10+ Backbone Secrets For Dna Stability
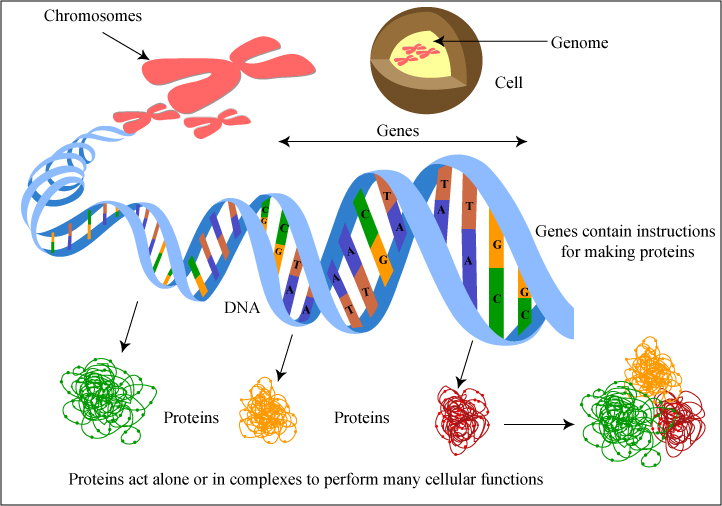
The intricate dance of molecular interactions within our cells is a testament to the awe-inspiring complexity of life. At the very heart of this complexity lies the double helix structure of DNA, whose stability is crucial for the integrity of genetic information. The backbone of DNA, comprising the sugar-phosphate molecules, plays a pivotal role in this stability. Delving into the secrets of how DNA maintains its stability reveals a fascinating world of biochemical interactions and structural adaptations. Here, we’ll explore over 10 backbone secrets that contribute to DNA stability, shedding light on the intricate mechanisms that ensure the faithful replication and transmission of genetic material.
1. Sugar-Phosphate Backbone Flexibility
The sugar-phosphate backbone of DNA is not as rigid as it might seem. It has a degree of flexibility that allows it to absorb and distribute the stresses of various interactions, such as those with proteins or other DNA segments. This flexibility is crucial for allowing DNA to unwind and rewind during processes like replication and transcription, without suffering structural damage.
2. Hydrogen Bonding Between Bases
While the sugar-phosphate backbone provides the structural framework of DNA, the stability of the double helix is also significantly contributed by the hydrogen bonding between the base pairs—adenine (A) with thymine (T), and guanine (G) with cytosine ©. These bonds, though individually weak, collectively provide substantial stability to the DNA molecule due to their sheer number and specific pairing, which optimizes the structural integrity of the double helix.
3. Major and Minor Grooves
The double helix structure of DNA is characterized by major and minor grooves, which are the result of the backbone’s configuration and the base pairing. These grooves are not just structural features; they are also functional, providing binding sites for proteins that interact with DNA, such as transcription factors and repair enzymes. The specific dimensions and chemical properties of these grooves can influence the binding affinity and specificity of these proteins, thereby affecting DNA stability and function.
4. Base Stacking Interactions
In addition to hydrogen bonding, the bases in DNA also interact through stacking forces. These are hydrophobic interactions between the planar, ring-shaped structures of the bases, which stabilize the double helix by minimizing contact with water. Base stacking interactions contribute to the stability of the DNA double helix, especially in the absence of hydrogen bonding, such as in certain regions of RNA structures.
5. Cationic Effects
Positively charged ions (cations) can have a significant impact on DNA stability. Cations like sodium (Na+) and magnesium (Mg2+) can neutralize the negative charges on the phosphate backbone, reducing electrostatic repulsion between the two strands of the DNA double helix. This can stabilize the structure and facilitate interactions with other molecules. However, an excessive concentration of cations can also destabilize DNA by promoting aggregation or altering its secondary structure.
6. Topological Constraints
The topological state of DNA, which includes its supercoiling and knotting, affects its stability and functionality. Supercoiling, for instance, can compact DNA, making it less accessible to enzymes but also potentially more stable in certain contexts. Topoisomerases are enzymes that help manage the topological state of DNA, relieving tensions and ensuring that DNA remains accessible for essential processes.
7. Nucleosome Formation
In eukaryotic cells, DNA wraps around histone proteins to form nucleosomes, which are fundamental units of chromatin. This wrapping not only compacts DNA but also influences its stability by protecting it from enzymatic degradation and mechanical stress. The formation of nucleosomes and higher-order chromatin structures is crucial for DNA stability and plays a key role in regulating gene expression.
8. DNA-Binding Proteins
A wide array of proteins interacts with DNA, influencing its stability and functionality. These include not just histones but also transcription factors, repair enzymes, and structural maintenance of chromosomes (SMC) proteins. Each type of protein has specific functions, from compacting DNA and regulating gene expression to repairing damages and ensuring proper segregation of DNA during cell division.
9. Environmental and Chemical Stability
The stability of DNA can be affected by environmental factors such as temperature, humidity, and the presence of chemicals. High temperatures, for example, can increase the kinetic energy of molecules, potentially destabilizing the hydrogen bonds between base pairs and causing denaturation. Chemical agents, such as those used in chemical mutagenesis, can also compromise DNA stability by directly altering its structure or by inducing mutations.
10. Repair Mechanisms
DNA is under constant threat from internal and external sources, including errors during replication, chemical mutagens, and ionizing radiation. The stability of DNA is thus reliant on a battery of repair mechanisms that can correct damage to the molecule. These mechanisms, including base excision repair, nucleotide excision repair, mismatch repair, and double-strand break repair, are essential for maintaining the integrity of genetic information.
11. Evolutionary Conservation
Lastly, the stability of DNA is also a testament to evolutionary forces that have shaped the molecule over billions of years. The conservation of the DNA double helix structure across nearly all forms of life on Earth reflects its adaptability and stability under a wide range of conditions. Evolutionary pressures have refined the chemical and physical properties of DNA, optimizing its stability for the transmission of genetic information.
What are the primary forces responsible for DNA stability?
+The primary forces include hydrogen bonding between the bases, base stacking interactions, and the electrostatic repulsion managed by the sugar-phosphate backbone, along with topological constraints and interactions with proteins.
How does the environment affect DNA stability?
+Environmental factors such as temperature, humidity, and the presence of chemicals can impact DNA stability. For instance, high temperatures can cause denaturation, while certain chemicals can lead to mutations or structural damage.
What is the role of nucleosomes in DNA stability?
+Nucleosomes, formed by DNA wrapping around histone proteins, help compact DNA and protect it from enzymatic degradation and mechanical stress, thus contributing to its overall stability and regulating gene expression.
In conclusion, the stability of DNA is a multifaceted phenomenon that relies on a combination of its structural attributes, the biochemical environment, and the dynamic interactions with a plethora of proteins. Understanding these factors not only provides insights into the fundamental biology of genetic material but also has implications for fields such as genetics, biotechnology, and medicine, where manipulating and preserving DNA stability are of paramount importance.


