10+ Ch3oh Secrets For Understanding Molecular Shape
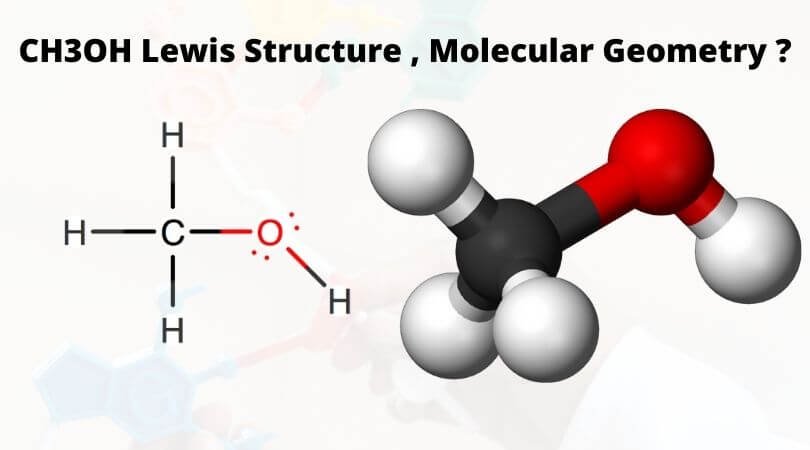
The molecular shape of CH3OH, commonly known as methanol, is a fundamental concept in chemistry that has significant implications for its physical and chemical properties. Understanding the molecular shape of CH3OH requires a deep dive into the realm of molecular geometry and the factors that influence it. In this article, we will explore 10+ secrets for understanding the molecular shape of CH3OH, providing a comprehensive overview of the subject.
To begin with, methanol is a polar molecule, consisting of a methyl group (CH3) attached to a hydroxyl group (OH). The molecular formula of methanol is CH3OH, and its molecular weight is approximately 32.04 g/mol. The molecular shape of CH3OH is tetrahedral, with the carbon atom at the center surrounded by three hydrogen atoms and one oxygen atom. However, the shape of the molecule is not perfectly tetrahedral due to the difference in electronegativity between the carbon and oxygen atoms.
Electronegativity and Bond Length: The electronegativity of the oxygen atom in methanol is significantly higher than that of the carbon atom, resulting in a shorter bond length between the carbon and oxygen atoms. This shorter bond length contributes to the bent or V-shape of the molecular geometry.
Hybridization and Orbitals: The carbon atom in methanol is sp3 hybridized, meaning that it has four equivalent orbitals that are oriented in a tetrahedral arrangement. The oxygen atom, on the other hand, has two lone pairs of electrons that occupy two of the sp3 hybrid orbitals, resulting in a bent shape.
Lone Pairs and Molecular Shape: The presence of lone pairs on the oxygen atom has a significant impact on the molecular shape of CH3OH. The lone pairs occupy more space than the bonding pairs, resulting in a bent or V-shape molecular geometry.
Dipole Moment and Polarity: Methanol is a polar molecule, with a significant dipole moment due to the difference in electronegativity between the carbon and oxygen atoms. The polarity of the molecule contributes to its physical and chemical properties, such as its boiling point and solubility.
Hydrogen Bonding and Intermolecular Forces: Methanol is capable of forming hydrogen bonds with other methanol molecules, resulting in stronger intermolecular forces. Hydrogen bonding is a critical factor in determining the physical properties of methanol, such as its boiling point and viscosity.
Molecular Orbital Theory: The molecular orbital theory provides a more detailed understanding of the molecular shape of CH3OH. The theory describes the distribution of electrons within the molecule and the formation of molecular orbitals.
VSEPR Theory and Molecular Geometry: The VSEPR (Valence Shell Electron Pair Repulsion) theory is a useful model for predicting the molecular shape of CH3OH. The theory states that the geometry of the molecule is determined by the repulsion between electron pairs.
Steric Effects and Molecular Shape: The steric effects of the methyl group and the hydroxyl group also play a role in determining the molecular shape of CH3OH. The size and shape of the groups can influence the bond angles and lengths, resulting in a bent or V-shape molecular geometry.
Quantum Mechanics and Molecular Shape: Quantum mechanics provides a more detailed understanding of the molecular shape of CH3OH, describing the behavior of electrons within the molecule and the formation of molecular orbitals.
Experimental Methods and Molecular Shape: Experimental methods, such as infrared spectroscopy and nuclear magnetic resonance (NMR) spectroscopy, can provide valuable information about the molecular shape of CH3OH. These methods can be used to determine the bond lengths, bond angles, and molecular geometry of the molecule.
In addition to these secrets, there are several other factors that can influence the molecular shape of CH3OH, including:
- Temperature and Pressure: Temperature and pressure can affect the molecular shape of CH3OH by altering the bond lengths and angles.
- Solvent Effects: The presence of a solvent can influence the molecular shape of CH3OH by altering the intermolecular forces and the molecular geometry.
- Concentration and Molecular Shape: The concentration of methanol can affect its molecular shape, with more concentrated solutions exhibiting stronger intermolecular forces and a more bent molecular geometry.
Understanding the molecular shape of CH3OH is critical for predicting its physical and chemical properties, such as its boiling point, solubility, and reactivity. The molecular shape of methanol is influenced by a combination of factors, including electronegativity, hybridization, lone pairs, dipole moment, hydrogen bonding, and steric effects.
The molecular shape of CH3OH is tetrahedral, with the carbon atom at the center surrounded by three hydrogen atoms and one oxygen atom. However, the shape of the molecule is not perfectly tetrahedral due to the difference in electronegativity between the carbon and oxygen atoms, resulting in a bent or V-shape molecular geometry.
Advantages and Disadvantages of Understanding Molecular Shape
| Advantages | Disadvantages |
|---|---|
| Predicting physical and chemical properties | Complexity of molecular geometry |
| Understanding reactivity and solubility | Difficulty in visualizing molecular shape |
| Designing new materials and applications | Limitations of experimental methods |

What is the molecular shape of CH3OH?
+The molecular shape of CH3OH is tetrahedral, with the carbon atom at the center surrounded by three hydrogen atoms and one oxygen atom. However, the shape of the molecule is not perfectly tetrahedral due to the difference in electronegativity between the carbon and oxygen atoms, resulting in a bent or V-shape molecular geometry.
What factors influence the molecular shape of CH3OH?
+The molecular shape of CH3OH is influenced by a combination of factors, including electronegativity, hybridization, lone pairs, dipole moment, hydrogen bonding, and steric effects.
Why is understanding the molecular shape of CH3OH important?
+Understanding the molecular shape of CH3OH is critical for predicting its physical and chemical properties, such as its boiling point, solubility, and reactivity.
In conclusion, the molecular shape of CH3OH is a complex and multifaceted concept that is influenced by a combination of factors. Understanding the molecular shape of methanol is critical for predicting its physical and chemical properties, and has significant implications for its applications in various fields. By exploring the secrets of the molecular shape of CH3OH, we can gain a deeper understanding of the underlying principles of molecular geometry and its importance in chemistry.



