10 Joules Units Explained Clearly
When delving into the realm of physics and energy, understanding the units of measurement is crucial. One of the fundamental units in the International System of Units (SI) for energy, work, and heat is the Joule (J). The Joule is named after James Prescott Joule, an English physicist who made significant contributions to the study of energy in the 19th century. In this explanation, we will break down the concept of 10 Joules, exploring what it represents and how it applies to various scenarios.
Definition of a Joule
A Joule is defined as the energy expended (or work done) when a force of one newton is applied over a distance of one meter. Mathematically, this can be represented as Joules (J) = Newtons (N) * Meters (m). This basic definition underscores the Joule’s role in quantifying energy in its various forms, whether it’s kinetic energy (the energy of motion), potential energy (stored energy), thermal energy, or any other form.
Understanding 10 Joules
10 Joules, therefore, represents a specific quantity of energy. To put this into perspective, consider a few examples:
Lifting an Object: If you were to lift a 1-kilogram object (approximately 2.2 pounds) upwards by 1 meter (about 3.3 feet) at a constant speed, you would expend approximately 9.8 Joules of energy, given that the force (weight) of the object is 9.8 Newtons (1 kg * 9.8 m/s^2, where g = 9.8 m/s^2). Thus, lifting the same object by 1.02 meters would require about 10 Joules of energy.
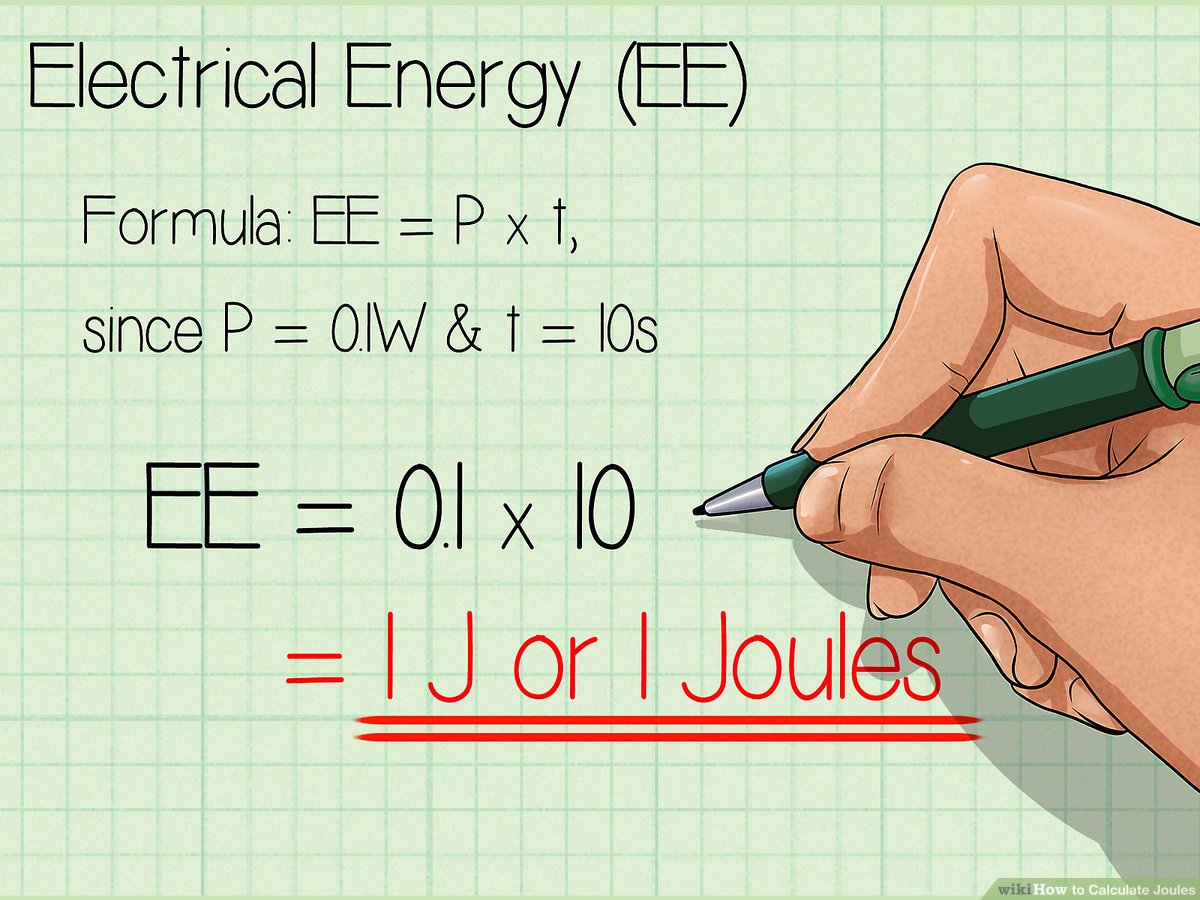
Energy Consumption: Many small electronic devices, like remote controls or small toys, operate at very low power consumption levels. For instance, a device consuming 5 watts of power would use 10 Joules of energy in 2 seconds (since Power (W) = Energy (J) / Time (s), rearranging for Energy gives us Energy = Power * Time, hence 5 W * 2 s = 10 J).
Thermal Energy: In terms of heat, 10 Joules could represent the amount of thermal energy required to change the temperature of a small amount of water. For example, to raise the temperature of 2.5 grams of water by 1 degree Celsius (or Kelvin), approximately 10.45 Joules of energy would be needed, considering water’s specific heat capacity is about 4.184 J/g°C.
Practical Applications and Examples
The concept of 10 Joules is not just theoretical but has practical implications in various fields:
Mechanical Engineering: In designing mechanical systems, understanding the energy required for different operations is crucial. For instance, calculating the energy needed to move parts of a machine or to overcome friction can be essential in optimizing system performance.
Electrical Engineering: When dealing with electrical circuits, the energy consumed or produced can be critical. For small-scale devices, calculating energy in Joules can help in optimizing battery life or energy efficiency.
Thermodynamics: In heating or cooling applications, whether it’s a small device like a water heater or a large system like a building’s HVAC, understanding energy requirements is vital for efficiency and performance.
Conclusion
In conclusion, 10 Joules represents a quantifiable amount of energy that can be applied in numerous contexts, from mechanical work to thermal energy. Understanding this unit and its implications can provide insights into the efficiency, performance, and potential of various systems and devices. Whether you’re a student of physics, an engineer, or simply someone curious about how the world works, grasping the concept of energy in its most fundamental units is invaluable.
Frequently Asked Questions
What is a Joule in simple terms?
+A Joule is a unit of energy or work, equivalent to the energy expended when a force of one newton is applied over a distance of one meter.
How much energy is 10 Joules?
+10 Joules can represent different amounts of work or energy depending on the context, such as lifting an object, powering a device, or changing the temperature of a substance.
What are practical applications of understanding Joules?
+Understanding Joules is crucial in various fields like mechanical engineering, electrical engineering, and thermodynamics, helping in designing efficient systems, calculating energy requirements, and optimizing performance.
By exploring the concept of 10 Joules and its applications, it becomes clear that energy, in all its forms, plays a fundamental role in our understanding and interaction with the world around us. Whether it’s the energy expended in physical work or the energy consumed by devices, each Joule counts in the grand scheme of efficiency, innovation, and sustainability.


