10+ Mn Electron Configuration Secrets Revealed
The electron configuration of an atom is a crucial concept in chemistry, as it determines the chemical properties of an element. The electron configuration is the arrangement of electrons in an atom, and it is typically represented using the Aufbau principle and the Pauli exclusion principle. In this article, we will delve into the secrets of the electron configuration of manganese (Mn), a transition metal with an atomic number of 25.
Introduction to Electron Configuration Before we dive into the specifics of manganese, it is essential to understand the basics of electron configuration. The electron configuration of an atom is the arrangement of electrons in the atom’s orbitals. The orbitals are the regions around the nucleus where the electrons are likely to be found. The electron configuration is typically represented using the Aufbau principle, which states that electrons fill the lowest available energy levels first. The Pauli exclusion principle, on the other hand, states that no two electrons in an atom can have the same set of quantum numbers.
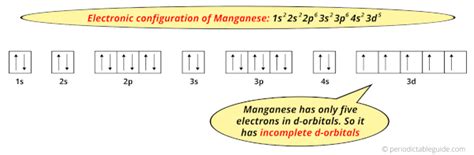
Mn Electron Configuration: A Deep Dive The electron configuration of manganese (Mn) is [Ar] 3d5 4s2. This means that the outermost energy level of the manganese atom contains five electrons in the 3d orbital and two electrons in the 4s orbital. The [Ar] notation indicates that the inner energy levels of the manganese atom are similar to those of argon, with a full outer energy level.
Understanding the 3d and 4s Orbitals To fully understand the electron configuration of manganese, it is essential to grasp the concept of the 3d and 4s orbitals. The 3d orbital is a type of atomic orbital that is shaped like a four-leaf clover. This orbital is responsible for the magnetic properties of manganese and is also involved in the formation of chemical bonds. The 4s orbital, on the other hand, is a spherical orbital that is lower in energy than the 3d orbital. The 4s orbital is typically filled before the 3d orbital, as it is lower in energy.
Why is the Mn Electron Configuration Important? The electron configuration of manganese is crucial in determining its chemical properties. The arrangement of electrons in the manganese atom determines how it will react with other elements. For example, the five electrons in the 3d orbital of manganese make it a highly reactive element, prone to forming ions with a +2 or +3 charge. The electron configuration also determines the magnetic properties of manganese, making it a ferromagnetic element.
Common Uses of Manganese Manganese is a versatile element with a wide range of applications. Some of the most common uses of manganese include:
- Steel Production: Manganese is used as an alloying element in steel production, as it improves the strength and hardness of steel.
- Batteries: Manganese is used in the production of batteries, particularly in the cathode of lithium-ion batteries.
- Pigments: Manganese is used as a pigment in the production of colors, such as manganese blue and manganese violet.
- Medicine: Manganese is used in medicine, particularly in the treatment of conditions such as osteoporosis and anemia.
Frequently Asked Questions
What is the electron configuration of manganese?
+The electron configuration of manganese is [Ar] 3d5 4s2.
Why is the electron configuration of manganese important?
+The electron configuration of manganese determines its chemical properties, including its reactivity and magnetic properties.
What are the common uses of manganese?
+Manganese is used in steel production, batteries, pigments, and medicine, among other applications.
Conclusion In conclusion, the electron configuration of manganese is a critical concept in understanding the chemical properties of this versatile element. The arrangement of electrons in the manganese atom determines its reactivity, magnetic properties, and other characteristics. By grasping the secrets of the manganese electron configuration, we can better appreciate the importance of this element in various industries and applications. Whether you are a chemist, engineer, or simply a curious learner, understanding the electron configuration of manganese is essential for unlocking its full potential.
Further Reading For those interested in learning more about the electron configuration of manganese and its applications, there are several resources available. Some recommended texts include:
- “Inorganic Chemistry” by Catherine Housecroft: This textbook provides a comprehensive introduction to inorganic chemistry, including the electron configuration of transition metals like manganese.
- “Chemistry of the Elements” by N.N. Greenwood: This book provides a detailed exploration of the chemistry of elements, including the electron configuration and properties of manganese.
- “Manganese: Its Occurrence, Metallurgy, and Uses” by the United States Geological Survey: This report provides an overview of the occurrence, production, and uses of manganese, including its electron configuration and chemical properties.
By delving deeper into the world of manganese and its electron configuration, you can gain a deeper understanding of the intricacies of chemistry and the importance of this element in various industries and applications.



