11 Carbon Element Electrons Revealed
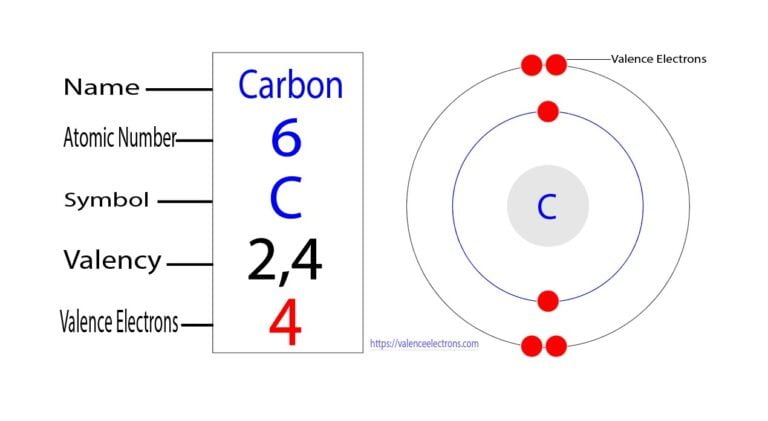
The carbon element, with its atomic number of 6, is a fundamental building block of life on Earth. At the heart of its chemical properties lies its electronic configuration, which plays a crucial role in determining its reactivity and ability to form a wide variety of compounds. In this context, understanding the electrons in a carbon atom is essential, particularly the number of electrons it has and how they are arranged.
Introduction to Electron Configuration

Electron configuration refers to the distribution of electrons within an atom’s orbitals. The Aufbau principle and the Pauli exclusion principle are key in understanding how electrons fill these orbitals. For carbon, with 6 electrons, the configuration is 1s² 2s² 2p². This means that the first energy level (or 1s orbital) is filled with 2 electrons, the second energy level’s s orbital (2s) is also filled with 2 electrons, and the remaining 2 electrons occupy the 2p orbitals.
The Role of Electrons in Chemical Bonding

The uniqueness of carbon’s electron configuration allows it to form an extraordinary number of compounds. The four valence electrons (2s² 2p²) are crucial for this ability. Carbon can form four covalent bonds, which is the basis for its central role in organic chemistry. This tetravalency of carbon enables it to create complex molecules, including biomolecules such as proteins, fats, and DNA.
Detailed Look at Carbon’s Electron Shells
To delve deeper into carbon’s electrons, it’s essential to understand the structure of its electron shells: - The first shell (or 1s orbital) contains 2 electrons. This shell is completely filled and does not participate in chemical bonding. - The second shell, which can hold up to 8 electrons, contains the remaining 4 electrons in carbon. This shell is divided into the 2s and 2p orbitals. The 2s orbital is filled with 2 electrons, and the 2p orbitals, which can hold a total of 6 electrons, contain the last 2 electrons.
Electron Movement and Chemical Reactivity
The arrangement and movement of electrons, particularly the valence electrons, determine carbon’s chemical reactivity. The ability of carbon atoms to share these electrons with other atoms (through covalent bonding) or to gain/lose electrons (forming ions) is at the heart of its chemical behavior.
Advanced Applications of Carbon’s Electronic Structure

Understanding the electronic structure of carbon has numerous practical applications: - Material Science: The knowledge of carbon’s electron configuration is crucial in the development of new materials, such as fullerenes, nanotubes, and graphene. These materials have unique properties due to the arrangement of carbon atoms and their electrons. - Chemical Synthesis: In organic synthesis, knowing how carbon electrons participate in chemical reactions allows chemists to design and synthesize complex molecules with specific properties. - Biological Systems: Carbon’s role in biological molecules (such as DNA, proteins, and carbohydrates) is foundational. The electron configuration of carbon allows it to form the backbone of these molecules, enabling the diversity of life on Earth.
Carbon’s Electrons in Different Forms
Carbon exists in several allotropes, including diamond, graphite, and amorphous carbon, each with unique properties derived from how the carbon atoms are arranged and bonded. Even in these different forms, the fundamental electronic configuration of carbon remains the same, but how the electrons participate in bonding varies: - Diamond: A rigid and transparent crystal where each carbon atom is bonded to four neighbors in a tetrahedral arrangement. - Graphite: A soft, slippery material where carbon atoms are arranged in layers, with each layer composed of hexagonal cells. Within each layer, carbon atoms are strongly bonded, but layers are weakly bonded to each other.
The Electron’s Role in Carbon’s Future Applications
As technology advances, the understanding of carbon’s electronic structure will become even more critical: - Quantum Computing: Carbon-based materials are being explored for their potential in quantum computing, where the manipulation of electron spins could form the basis of quantum bits (qubits). - Energy Storage: The development of advanced carbon materials for batteries and supercapacitors relies on understanding how electrons interact with these materials at the atomic and molecular level.
Frequently Asked Questions
Why is carbon so versatile in forming compounds?
+Carbon's versatility stems from its unique electron configuration, allowing it to form four covalent bonds and thus create a wide variety of molecules, from simple hydrocarbons to complex biomolecules.
How do the electrons in a carbon atom contribute to its ability to form long chains and rings?
+The arrangement of electrons, particularly the four valence electrons, allows carbon atoms to bond with each other in various configurations, including linear chains, branched chains, and rings, by forming covalent bonds.
What role does the electron configuration play in the differences between diamond, graphite, and fullerenes?
+The electron configuration itself remains the same across these allotropes. However, the difference in how carbon atoms are arranged and bonded in each form (due to the interaction of electrons in bonding) results in their distinct properties.
Conclusion
The electronic structure of carbon, with its 6 electrons arranged in a specific configuration, underpins the element’s unique chemical properties and its central role in life and technology. Understanding the behavior of these electrons is essential for advancing our knowledge of chemistry, materials science, and biological systems. As research continues to explore the potential of carbon-based materials and molecules, the depth of our understanding of carbon’s electrons will remain foundational.