12+ Ammonia Conjugate Base Secrets For Chemistry Mastery
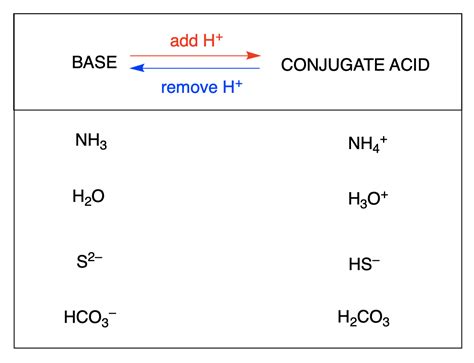
Understanding ammonia and its conjugate base is fundamental to mastering chemistry, particularly in the realm of acid-base chemistry. Ammonia (NH3) is a weak base that, when dissolved in water, forms ammonium hydroxide (NH4OH), which then dissociates into ammonium ions (NH4+) and hydroxide ions (OH-). The conjugate base of ammonia is the amide ion (NH2-), which plays a crucial role in various chemical reactions and biological processes.
1. Definition and Formation
The conjugate base of ammonia is formed when ammonia accepts a proton (H+), turning into its conjugate acid, ammonium (NH4+). Conversely, when ammonium donates a proton, it reverts to ammonia, and in a basic solution, ammonia can accept a proton to form ammonium, but in a more basic context, ammonia itself can act as an acid, donating a proton to form the amide ion (NH2-).
2. Chemical Properties
The amide ion (NH2-) is a strong base. It is the conjugate base of a weak base (ammonia), which means it is more basic than ammonia itself. This property makes the amide ion highly reactive in the presence of acids, readily accepting protons to revert to ammonia.
3. Biological Significance
In biological systems, ammonia and its conjugate base are involved in various metabolic pathways. For instance, in the urea cycle, ammonia is converted into urea, which is then excreted. This process is crucial for detoxifying ammonia, which is toxic to the nervous system in high concentrations.
4. pH and Buffer Solutions
Ammonia and its conjugate acid, ammonium, form a buffer solution that resists changes in pH. This buffer system is vital in maintaining the acid-base balance in the human body and in many chemical processes.
5. Synthetic Applications
The amide ion is used in organic synthesis as a strong nucleophile. Its ability to donate a pair of electrons makes it useful in forming new bonds, particularly in the synthesis of amides and other nitrogen-containing compounds.
6. Environmental Impact
Ammonia is used extensively in agriculture as a fertilizer. However, its overuse can lead to environmental problems, such as eutrophication in water bodies, where excess nitrogen promotes excessive plant growth, depleting the oxygen and harming aquatic life.
7. Historical Development
The understanding of ammonia and its conjugate base has evolved over centuries, with early chemists recognizing ammonia’s basic properties. The development of modern acid-base theories, such as Bronsted-Lowry and Lewis theories, has provided a deeper understanding of these compounds’ behavior.
8. Industrial Applications
Ammonia is a key feedstock in the production of many chemicals, including nitric acid, urea, and ammonium salts. These compounds are used in various industries, such as textiles, pharmaceuticals, and food production.
9. Acid-Base Theories
According to the Bronsted-Lowry theory, ammonia acts as a base by accepting a proton, while its conjugate base, the amide ion, is considered a strong base due to its ability to readily accept protons. The Lewis theory, on the other hand, defines bases as electron pair donors, which aligns with the amide ion’s behavior.
10. Toxicity and Safety
Ammonia itself is toxic and can cause severe health issues upon exposure, including respiratory distress and skin burns. Handling ammonia requires proper safety equipment and ventilation to prevent accidents.
11. Chemical Reactions
Ammonia participates in numerous chemical reactions, including the Haber-Bosch process for producing ammonia from nitrogen and hydrogen, which is a crucial step in fertilizer production. The amide ion also reacts with acids to form amides, a class of compounds found in many biological molecules and synthetic materials.
12. Analytical Detection
Detecting ammonia and its conjugate base involves various analytical techniques, such as titration, spectroscopy, and chromatography. These methods are essential in monitoring environmental pollution, controlling industrial processes, and diagnosing medical conditions related to ammonia metabolism.
13. Future Research Directions
Research into ammonia and its conjugate base continues, with focuses on more efficient synthesis methods, environmental impact mitigation, and medical applications. For instance, developing more sustainable production methods for ammonia could significantly reduce the environmental footprint of the agricultural and chemical industries.
FAQ Section
What is the conjugate base of ammonia?
+The conjugate base of ammonia is the amide ion (NH2-), which is formed when ammonia donates a proton.
Why is ammonia considered a weak base?
+Ammonia is considered a weak base because it does not completely dissociate in water to produce hydroxide ions. Instead, it forms an equilibrium mixture with its conjugate acid, ammonium.
What are some industrial applications of ammonia?
+Ammonia is used in the production of nitric acid, urea, and ammonium salts, among other chemicals, which are vital in various industries such as textiles, pharmaceuticals, and agriculture.
In conclusion, mastering the concepts related to ammonia and its conjugate base is crucial for a deep understanding of chemistry and its applications. From biological significance to industrial processes, the role of ammonia and the amide ion is multifaceted, reflecting the complexity and interconnectedness of chemical principles in both natural and synthetic contexts.

