8 Inner Transition Metals Secrets To Improve Chemistry Grades
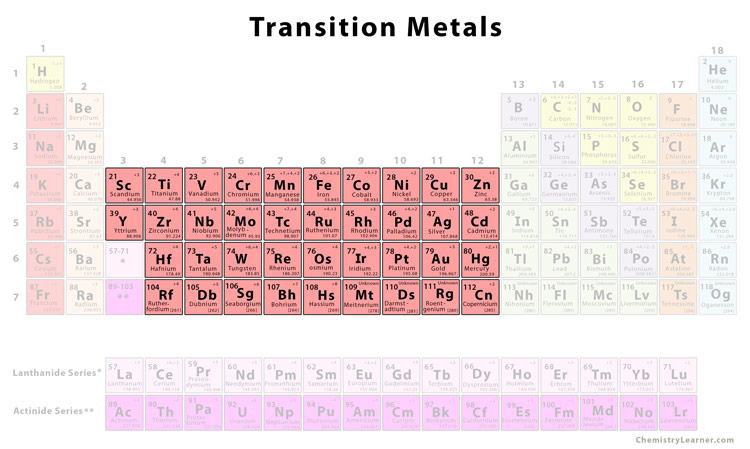
Delving into the realm of inner transition metals can be a daunting task for many chemistry students. These elements, which include the lanthanides and actinides, possess unique properties that set them apart from other metals. Understanding these properties and how inner transition metals behave is crucial for achieving success in chemistry. Here, we will explore eight secrets to mastering inner transition metals, helping you improve your chemistry grades and deepen your understanding of these fascinating elements.
1. Electronic Configuration: The Key to Properties
The electronic configuration of inner transition metals is foundational to understanding their chemical properties. These metals have partially filled f orbitals, which influence their ability to form ions, their magnetic properties, and their participation in chemical reactions. For instance, the lanthanides exhibit a gradual decrease in atomic and ionic radii as you move across the series, a phenomenon known as the lanthanide contraction. This contraction affects the chemical properties of these elements, such as their ability to form complexes.
2. Oxidation States and Stability
Inner transition metals are known for their ability to exhibit multiple oxidation states. This ability stems from the fact that the f orbitals are buried deep within the electron cloud, making it relatively easy to lose or gain electrons. However, the stability of these oxidation states varies, with certain states being more stable than others due to the half-filled or completely filled orbitals. For example, in the lanthanides, the +3 oxidation state is the most common and stable due to the tendency to achieve a stable f^0 or f^7 configuration.
3. Magnetic Properties
The magnetic properties of inner transition metals are another area of interest. These elements can exhibit paramagnetic, ferromagnetic, or antiferromagnetic properties depending on the number of unpaired electrons in their orbitals. The lanthanides, in particular, are known for their strong magnetic properties, which find applications in technologies such as magnetic resonance imaging (MRI) machines and high-temperature superconductors.
4. Coordination Chemistry
Inner transition metals form a wide range of complexes due to their ability to exhibit multiple oxidation states and coordinate with various ligands. The stability and geometry of these complexes are influenced by the size of the metal ion, the charge density, and the nature of the ligand. For instance, complexes of lanthanides with certain ligands can exhibit luminescent properties, finding applications in areas such as biomedical research and display technologies.
5. Lanthanide Contraction
The lanthanide contraction, mentioned earlier, has significant implications for the properties of inner transition metals. This phenomenon leads to elements in the third transition series (like gold and platinum) having similar atomic radii to those of elements in the second transition series (such as silver and palladium). This contraction affects not only the size of the ions but also their chemical behavior, influencing their reactivity and complexation reactions.
6. Actinides: Radioactivity and Reactivity
The actinide series, which includes elements like uranium and plutonium, is characterized by radioactivity. The actinides exhibit a range of oxidation states, with some being more stable than others. Their reactivity is also notable, with applications in nuclear energy and weaponry. However, handling these elements requires careful consideration due to their radioactive nature and potential environmental impact.
7. Separation and Purification Techniques
Given the similarities in chemical properties among inner transition metals, their separation and purification can be challenging. Techniques such as ion exchange chromatography, solvent extraction, and fractional crystallization are commonly employed to separate and purify these elements. Understanding the principles behind these techniques is essential for any application involving the use of inner transition metals.
8. Applications and Future Perspectives
Inner transition metals have a wide range of applications, from catalysts in chemical reactions to components in electronic devices, and from biomedical research tools to energy storage materials. Their unique properties make them indispensable in many technologies. As research continues, new applications are emerging, such as in the development of more efficient solar cells, advanced magnets for wind turbines, and safer nuclear fuels.
Conclusion
Mastering the secrets of inner transition metals requires a deep understanding of their electronic configurations, oxidation states, magnetic properties, coordination chemistry, and applications. By grasping these concepts, students of chemistry can not only improve their grades but also contribute to the development of new technologies and innovations that rely on these fascinating elements. Whether in the field of energy, medicine, or materials science, inner transition metals play a critical role, and their study represents a rewarding and challenging journey into the heart of chemistry.
FAQ Section
What are the primary challenges in studying inner transition metals?
+The primary challenges include their complex electronic configurations, the need for advanced techniques for their separation and purification, and understanding their varied oxidation states and reactivity.
How do inner transition metals contribute to technological advancements?
+They are crucial in the development of advanced materials, such as high-temperature superconductors, efficient catalysts, and components for renewable energy technologies, due to their unique magnetic, optical, and electrical properties.
What is the significance of the lanthanide contraction in chemistry?
+The lanthanide contraction affects the chemical properties of lanthanides, influencing their reactivity, complexation behavior, and the stability of their compounds. It also has practical implications for the separation and purification of these elements.
How do the magnetic properties of inner transition metals make them useful?
+The strong magnetic properties of these elements make them useful in applications such as magnetic resonance imaging (MRI), in the production of permanent magnets, and in data storage technologies.
What role do inner transition metals play in the field of nuclear energy?
+Actinides, a series of inner transition metals, are crucial in nuclear reactors as fuel (e.g., uranium-235) and in the development of new, safer nuclear fuels. Their radioactivity also finds applications in medicine and industry.
How are inner transition metals used in biomedical research and applications?
+They are used in diagnostic tools, therapeutic agents, and in research due to their luminescent properties (e.g., lanthanide complexes in microscopy) and their ability to be used as contrast agents in MRI.
In exploring the complex and fascinating world of inner transition metals, one delves into a realm of unique properties, diverse applications, and ongoing research. The mastery of these elements not only enhances one’s understanding of chemistry but also opens doors to innovative technologies and solutions, making their study both rewarding and essential for the advancement of science and technology.