Bef2 Lewis Structure
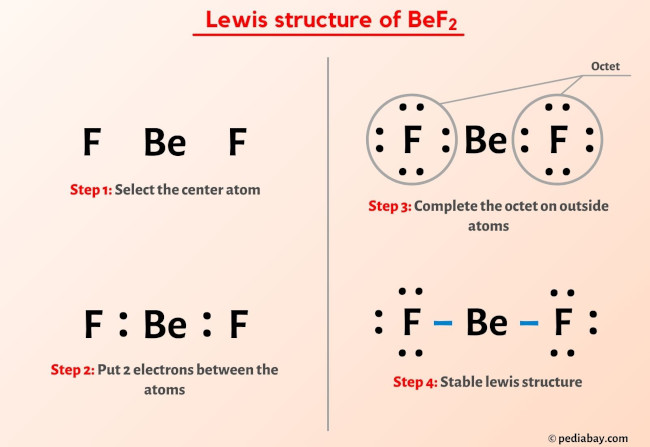
The Lewis structure of a molecule is a two-dimensional representation that shows the arrangement of atoms, their bonds, and lone pairs in a molecule. To draw the Lewis structure of BeF2, we need to follow a series of steps:
Determine the total number of valence electrons: Beryllium (Be) is in Group 2 of the periodic table, so it has 2 valence electrons. Fluorine (F) is in Group 17, so each fluorine atom has 7 valence electrons. Since there are two fluorine atoms in BeF2, the total number of valence electrons from fluorine is 2 * 7 = 14. Adding the valence electrons from beryllium gives a total of 2 (from Be) + 14 (from two F atoms) = 16 valence electrons.
Choose a central atom: Beryllium will be the central atom because it is less electronegative than fluorine. In general, the least electronegative atom is placed in the center.
Draw single bonds to the surrounding atoms: Draw single bonds from the central beryllium atom to each of the two fluorine atoms. Each single bond represents 2 electrons, so 4 electrons are used in the bonds between Be and the two F atoms.
Distribute the remaining electrons: After forming the single bonds, we have used 4 electrons, leaving 16 - 4 = 12 electrons to distribute around the atoms to satisfy the octet rule for each atom. The octet rule states that an atom will try to have 8 electrons in its outermost shell.
Complete the octet for each fluorine atom: Each fluorine atom already has 1 single bond (2 electrons) to beryllium, so we distribute the remaining electrons around the fluorine atoms to give each an octet. Since each fluorine needs 6 more electrons to achieve an octet (8 - 2 = 6), and we have 12 electrons to distribute, each fluorine atom can have 3 pairs of electrons (6 electrons), fulfilling the octet rule for both fluorine atoms.
Complete the octet for the beryllium atom: With the 4 electrons used in single bonds and no more electrons available, the beryllium atom ends up with 4 electrons (2 from each single bond), fulfilling its requirement for an octet due to its position in the periodic table and the nature of its bonding with fluorine. However, in this context, following traditional Lewis structure construction, we acknowledge that beryllium, having only 4 electrons, technically doesn’t achieve a full octet like the fluorines do, which is consistent with the electron-deficient nature of beryllium in BeF2.
The resulting Lewis structure of BeF2 shows beryllium bonded to two fluorine atoms with single bonds and each fluorine atom having three lone pairs of electrons. This arrangement satisfies the octet rule for the fluorine atoms and reflects the electron configuration of the beryllium atom in this molecule.
In conclusion, the Lewis structure of BeF2 is a straightforward representation once the steps to draw it are understood, providing insight into the molecule’s bonding and geometry.
Steps to Draw the Lewis Structure of BeF2:
- Determine the total number of valence electrons.
- Choose a central atom based on electronegativity.
- Draw single bonds to the surrounding atoms.
- Distribute the remaining electrons to fulfill the octet rule for each atom.
Understanding the Lewis structure of molecules like BeF2 is crucial for predicting their chemical properties and behaviors, including reactivity and molecular geometry, which are essential concepts in chemistry.
What is the shape of the BeF2 molecule?
+The BeF2 molecule has a linear shape due to the presence of two bonding pairs of electrons around the central beryllium atom and no lone pairs, resulting in a 180-degree bond angle between the Be-F bonds.
Why is beryllium the central atom in BeF2?
+Beryllium is the central atom because it is less electronegative than fluorine. In Lewis structures, the least electronegative atom is typically placed in the center.
By analyzing the Lewis structure of BeF2, one can gain insights into its molecular characteristics and how these influence its physical and chemical properties.
Pros and Cons of the Lewis Structure of BeF2:
- Pros: The Lewis structure effectively predicts the linear geometry of BeF2 and provides a clear representation of the molecule's electron configuration.
- Cons: The Lewis structure might oversimplify the molecule's electron distribution and does not account for the detailed molecular orbital theory, which offers a more comprehensive understanding of molecular bonding.
The study of Lewis structures, including that of BeF2, underscores the importance of understanding molecular geometry and electron distribution in predicting chemical behavior and properties.