Benzene Peak Ir
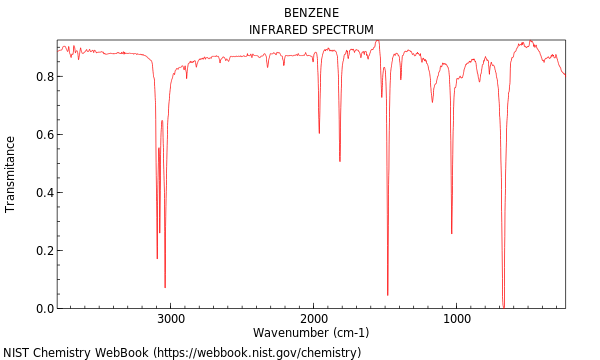
In the realm of organic chemistry, the analysis of aromatic compounds holds significant importance, and one of the most iconic aromatic hydrocarbons is benzene. The identification and quantification of benzene in various samples often rely on spectroscopic techniques, with infrared (IR) spectroscopy being a powerful tool. The “Benzene Peak” in IR spectroscopy refers to the characteristic absorption bands that provide valuable insights into the molecular structure and functional groups present in benzene and its derivatives.
Unlocking the Secrets of Benzene’s IR Spectrum

The Fundamentals of IR Spectroscopy
Infrared spectroscopy is a versatile analytical technique that probes the vibrational modes of molecules by measuring their absorption of infrared light. When infrared radiation interacts with a sample, it can cause the bonds within the molecule to vibrate at specific frequencies, which are characteristic of the type of bond and its environment. These vibrations are quantized, meaning they occur at discrete energy levels, and the corresponding absorption peaks in the IR spectrum provide a unique fingerprint for each compound.
Benzene’s Unique IR Signature
Benzene (C6H6) is a planar, cyclic molecule with a distinctive structure consisting of a ring of six carbon atoms, each with one hydrogen atom attached. This arrangement results in a set of specific vibrational modes that give rise to characteristic peaks in its IR spectrum. The most prominent feature is the C-H stretching vibration, which typically appears in the region of 3000-3100 cm^-1. This peak is a hallmark of aromatic compounds and is often used as a diagnostic tool for their identification.
Deconstructing the Benzene IR Spectrum
The IR spectrum of benzene reveals a wealth of information beyond the prominent C-H stretch. Here’s a breakdown of key features:
C-H Bending Vibrations (1000-1600 cm^-1): This region is characterized by a series of peaks corresponding to the bending vibrations of the C-H bonds. The exact positions and intensities of these peaks can vary depending on the substitution pattern of the benzene ring.
C-C Stretching Vibrations (1400-1600 cm^-1): The aromatic C-C bonds in benzene give rise to absorption bands in this region. These peaks are often less intense compared to the C-H vibrations but provide valuable information about the ring structure.
Out-of-Plane C-H Bending (700-900 cm^-1): This region is particularly useful for distinguishing between different substitution patterns on the benzene ring. Mono-substituted benzenes typically exhibit a strong peak around 750-800 cm^-1, while di-substituted compounds may show additional peaks depending on the relative positions of the substituents.
Applications and Practical Considerations
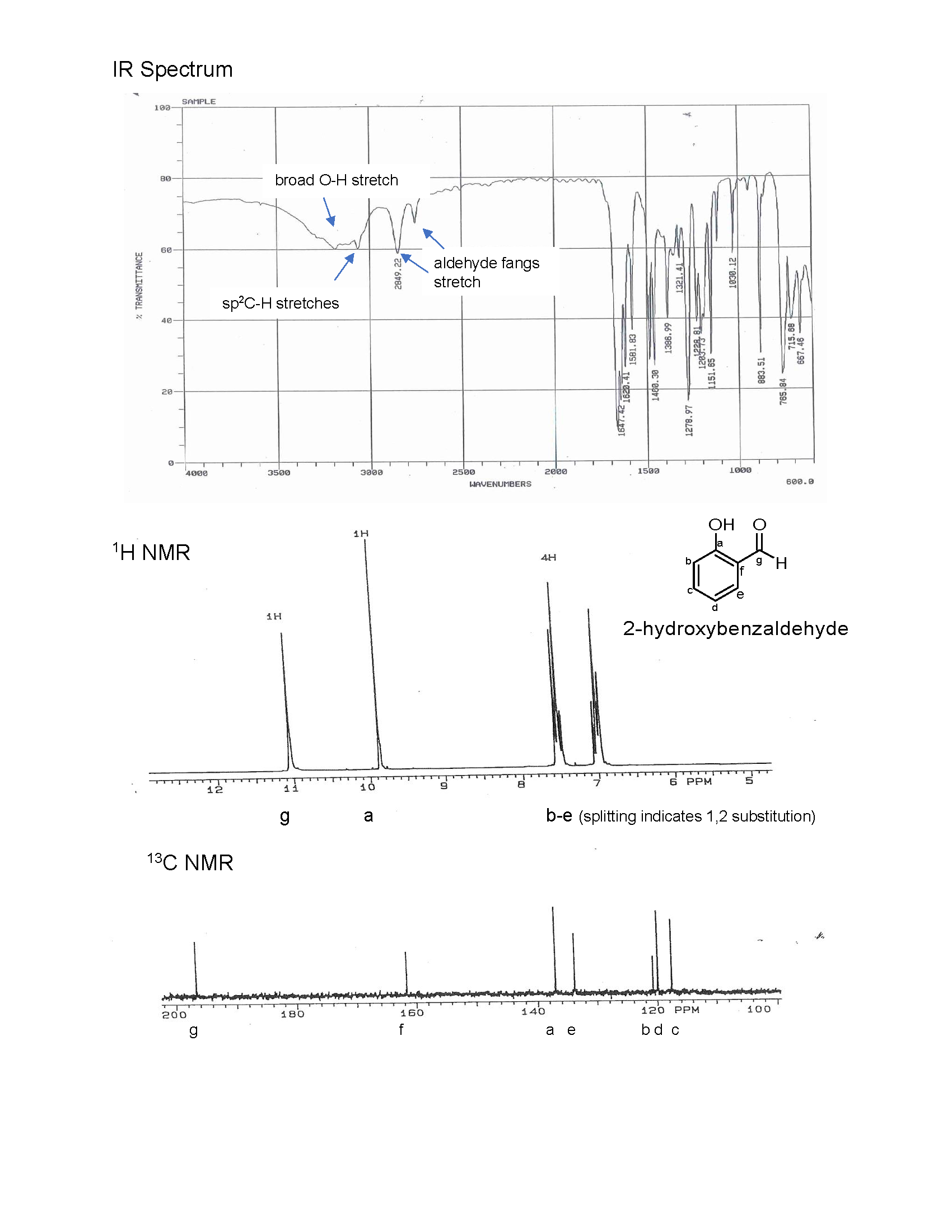
Identifying Benzene in Complex Mixtures
In real-world scenarios, benzene is often found in mixtures with other hydrocarbons, making its identification and quantification crucial in various industries. IR spectroscopy, coupled with advanced data analysis techniques, enables the detection of benzene even in complex matrices. For instance, in the petrochemical industry, IR spectroscopy is used to monitor benzene levels in gasoline and other petroleum products to ensure compliance with safety regulations.
Quantitative Analysis and Calibration
The intensity of the benzene peak in IR spectroscopy can be used for quantitative analysis, provided that appropriate calibration curves are established. This involves creating a series of standard solutions with known concentrations of benzene and measuring their IR spectra. The relationship between peak intensity and concentration allows for the determination of benzene levels in unknown samples.
Limitations and Interferences
While IR spectroscopy is a powerful tool, it is not without limitations. One challenge is the potential for overlapping peaks, especially in complex mixtures. In such cases, advanced techniques like Fourier-transform infrared (FTIR) spectroscopy and multivariate data analysis can help resolve individual components. Additionally, the presence of strong absorbers, such as water, can interfere with the detection of benzene peaks, requiring careful sample preparation and the use of appropriate reference spectra.
Comparative Analysis: Benzene vs. Other Aromatics
To fully appreciate the uniqueness of benzene’s IR spectrum, a comparative analysis with other aromatic compounds is insightful.
| Compound | C-H Stretch (cm^-1) | C-C Stretch (cm^-1) | Out-of-Plane C-H Bend (cm^-1) |
|---|---|---|---|
| Benzene (C6H6) | 3030 | 1450, 1600 | 750-800 |
| Toluene (C7H8) | 3050, 3020 | 1490, 1600 | 800-850 |
| Xylene (C8H10) | 3080, 3050, 3020 | 1490, 1600 | 800-900 |
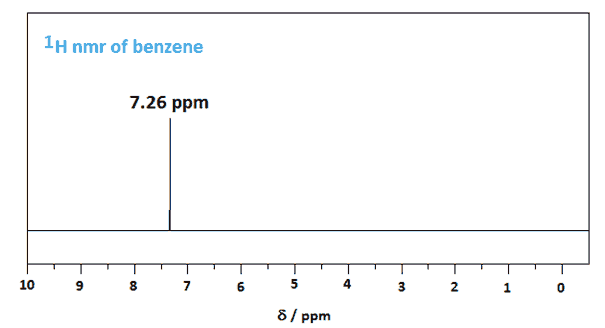
Historical Perspective: The Evolution of IR Spectroscopy
The development of IR spectroscopy as a tool for benzene analysis has a rich history. In the early 20th century, the pioneering work of scientists like Sir George Stokes and William Herschel laid the foundation for understanding infrared radiation and its interaction with matter. However, it was not until the mid-20th century that IR spectroscopy became a practical analytical technique with the advent of modern instruments.
Over the years, advancements in technology have led to the development of more sophisticated IR spectrometers, such as FTIR instruments, which offer improved sensitivity, resolution, and data processing capabilities. These innovations have further solidified IR spectroscopy’s role in benzene analysis and its applications in various scientific disciplines.
Future Trends: Pushing the Boundaries of IR Spectroscopy
As technology continues to evolve, the future of IR spectroscopy in benzene analysis looks promising. Here are some emerging trends:
Hyperspectral Imaging: This technique combines spectroscopy and imaging, allowing for the simultaneous acquisition of spectral and spatial information. It enables the visualization of benzene distribution in complex samples, such as biological tissues or environmental matrices.
Machine Learning Integration: Advanced data analysis techniques, including machine learning algorithms, are being employed to extract more information from IR spectra. These methods can enhance the accuracy of benzene quantification and enable the identification of subtle spectral patterns.
Portable and Field-Deployable Instruments: The development of compact and portable IR spectrometers is making benzene analysis more accessible in remote locations and field settings, such as environmental monitoring sites or industrial facilities.
FAQ Section
How does the presence of substituents on the benzene ring affect its IR spectrum?
+Substituents on the benzene ring can significantly influence its IR spectrum. Electron-donating groups (e.g., -OH, -NH2) can cause a shift in the C-H stretching vibration to lower wavenumbers, while electron-withdrawing groups (e.g., -NO2, -COOH) may result in a shift to higher wavenumbers. Additionally, the out-of-plane C-H bending region can provide information about the substitution pattern, with different peaks appearing for ortho, meta, and para substitutions.
Can IR spectroscopy distinguish between benzene and its isotopologues (e.g., deuterated benzene)?
+Yes, IR spectroscopy can differentiate between benzene and its isotopologues. The C-D stretching vibration in deuterated benzene (C6D6) appears at a lower wavenumber compared to the C-H stretch in regular benzene. This difference is due to the stronger bond strength of C-D compared to C-H, resulting in a lower vibrational frequency.
What are the advantages of using FTIR spectroscopy for benzene analysis?
+Fourier-transform infrared (FTIR) spectroscopy offers several advantages for benzene analysis. It provides higher sensitivity, improved signal-to-noise ratio, and faster data acquisition compared to traditional dispersive IR spectrometers. FTIR also enables the collection of a complete spectrum in a single measurement, facilitating the analysis of complex mixtures and the resolution of overlapping peaks.
How can IR spectroscopy be used to detect benzene in the atmosphere?
+Atmospheric monitoring of benzene often employs remote sensing techniques, such as open-path FTIR spectroscopy. This method involves measuring the infrared radiation absorbed by the atmosphere over a long path length, allowing for the detection of benzene and other volatile organic compounds. By comparing the measured spectrum with reference spectra, analysts can quantify benzene concentrations in the air.
What sample preparation techniques are essential for accurate benzene analysis by IR spectroscopy?
+Sample preparation is critical for obtaining accurate IR spectra. For liquid samples, proper dilution and the use of suitable solvents are essential to avoid concentration effects and solvent interference. Solid samples may require grinding and mixing with a non-absorbing material, such as KBr, to create a homogeneous mixture. Additionally, ensuring the sample is free from moisture is crucial, as water can interfere with the benzene peak region.
In conclusion, the “Benzene Peak” in IR spectroscopy is a powerful tool for identifying and analyzing this essential aromatic compound. From its unique vibrational signature to its applications in various industries, the study of benzene’s IR spectrum continues to evolve, driven by technological advancements and innovative research. As chemists and analysts, understanding the nuances of benzene’s IR behavior empowers us to make informed decisions, ensure product quality, and contribute to a safer and more sustainable world.