Benzoic Acid Intermolecular Forces
Introduction
Benzoic acid, a simple aromatic carboxylic acid with the formula C₆H₅COOH, is a fascinating molecule that exhibits a variety of intermolecular forces. These forces play a crucial role in determining its physical properties, such as melting point, boiling point, and solubility. In this article, we will delve into the world of benzoic acid’s intermolecular forces, exploring their types, strengths, and implications.
Types of Intermolecular Forces in Benzoic Acid
Benzoic acid experiences several types of intermolecular forces, including:
- Hydrogen Bonding: The carboxyl group (-COOH) in benzoic acid can form strong hydrogen bonds with neighboring molecules. This occurs when the hydrogen atom of one molecule is attracted to the oxygen atom of another molecule, resulting in a relatively strong intermolecular force.
- Dipole-Dipole Interactions: The polar nature of the C=O and O-H bonds in benzoic acid gives rise to permanent dipole moments. These dipoles can interact with each other, leading to dipole-dipole forces between molecules.
- London Dispersion Forces (LDF): Also known as van der Waals forces, LDFs are weak intermolecular forces that arise due to temporary fluctuations in electron density. These forces are present in all molecules, including benzoic acid, and become more significant as the size and complexity of the molecule increase.
- π-π Stacking: The aromatic ring in benzoic acid can engage in π-π stacking interactions with neighboring rings. This occurs when the π electrons of one ring interact with those of another, resulting in a weak intermolecular force.
Strengths and Implications of Intermolecular Forces
The strengths of these intermolecular forces in benzoic acid can be ranked as follows:
- Hydrogen Bonding > Dipole-Dipole > π-π Stacking > London Dispersion Forces
This ranking has significant implications for benzoic acid’s physical properties:
- Melting and Boiling Points: The strong hydrogen bonding in benzoic acid results in a relatively high melting point (122°C) and boiling point (249°C) compared to other organic compounds of similar molecular weight.
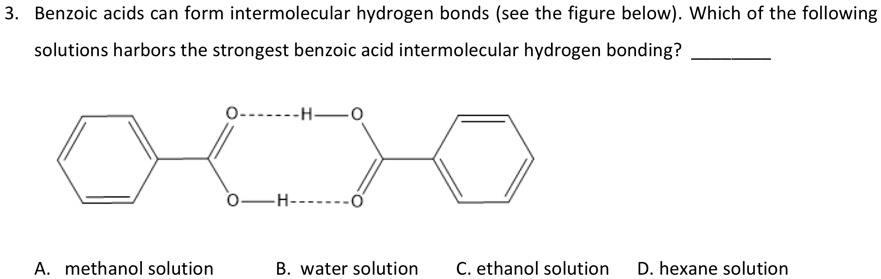
- Solubility: Benzoic acid is soluble in polar solvents like water due to its ability to form hydrogen bonds with the solvent molecules. However, it is only sparingly soluble in nonpolar solvents like hexane, where hydrogen bonding is not possible.
- Crystal Structure: The intermolecular forces in benzoic acid also influence its crystal structure. The molecule adopts a planar conformation in the solid state, with the carboxyl group participating in an extensive network of hydrogen bonds.
Comparative Analysis with Other Carboxylic Acids
To better understand the role of intermolecular forces in benzoic acid, let’s compare it with other carboxylic acids:
| Compound | Melting Point (°C) | Boiling Point (°C) | Solubility in Water (g/100mL) |
|---|---|---|---|
| Formic Acid (HCOOH) | 8.4 | 100.8 | Miscible |
| Acetic Acid (CH₃COOH) | 16.6 | 118.1 | Miscible |
| Benzoic Acid (C₆H₅COOH) | 122 | 249 | 0.34 (25°C) |
As shown in the table, benzoic acid has a significantly higher melting and boiling point compared to formic and acetic acids. This can be attributed to the additional intermolecular forces present in benzoic acid, such as π-π stacking and London dispersion forces, which are absent in the smaller carboxylic acids.
Practical Applications
The understanding of benzoic acid’s intermolecular forces has practical applications in various fields:
- Pharmaceuticals: Benzoic acid is used as a preservative in pharmaceuticals due to its antimicrobial properties. Its solubility and intermolecular forces play a crucial role in determining its effectiveness.
- Food Industry: Benzoic acid is used as a food preservative, particularly in acidic foods like pickles and sauces. Its intermolecular forces influence its distribution and stability in these products.
- Organic Synthesis: Benzoic acid is a common starting material in organic synthesis. Its reactivity and intermolecular forces affect the outcome of chemical reactions.
Future Trends and Research Directions
As research in the field of intermolecular forces continues to advance, new insights into benzoic acid’s behavior are likely to emerge. Some potential areas of future research include:
- Computational Modeling: Advanced computational methods can be used to simulate the intermolecular forces in benzoic acid and predict its properties under different conditions.
- Nanomaterials: The incorporation of benzoic acid into nanomaterials may lead to new applications in fields like electronics and energy storage.
- Biomolecular Interactions: Investigating the role of benzoic acid’s intermolecular forces in biomolecular interactions, such as protein-ligand binding, could provide valuable insights into drug design and discovery.
FAQ Section
What is the primary intermolecular force in benzoic acid?
+The primary intermolecular force in benzoic acid is hydrogen bonding, which occurs between the carboxyl group (-COOH) of one molecule and the oxygen atom of another molecule.
How do intermolecular forces affect the solubility of benzoic acid?
+Intermolecular forces, particularly hydrogen bonding, play a crucial role in determining the solubility of benzoic acid. The molecule is soluble in polar solvents like water, where it can form hydrogen bonds with the solvent molecules, but only sparingly soluble in nonpolar solvents like hexane.
What is the role of π-π stacking in benzoic acid?
+π-π stacking is a weak intermolecular force that occurs between the aromatic rings of benzoic acid molecules. It contributes to the overall stability of the crystal structure and influences the molecule's physical properties, such as melting and boiling points.
How does benzoic acid's crystal structure relate to its intermolecular forces?
+Benzoic acid's crystal structure is characterized by a layered arrangement of molecules, with each layer consisting of a herringbone pattern. The carboxyl groups are oriented in a way that maximizes hydrogen bonding between layers, reflecting the importance of this intermolecular force in determining the molecule's structure.
What are the practical applications of understanding benzoic acid's intermolecular forces?
+Understanding benzoic acid's intermolecular forces has practical applications in fields like pharmaceuticals, food industry, and organic synthesis. It helps optimize the use of benzoic acid as a preservative, predict its reactivity in chemical reactions, and design new materials with tailored properties.
Conclusion
In conclusion, the intermolecular forces in benzoic acid are a complex and fascinating topic that spans multiple disciplines. From its physical properties to its practical applications, a thorough understanding of these forces is essential for unlocking the full potential of this versatile molecule. As research continues to advance, we can expect to gain even deeper insights into the behavior of benzoic acid and its implications in various fields.
By balancing these factors and leveraging our understanding of benzoic acid’s intermolecular forces, we can harness its unique properties and develop innovative solutions to real-world problems. Whether in pharmaceuticals, food science, or materials chemistry, benzoic acid remains a molecule of great interest and importance.