C6h12o6 Polar Or Nonpolar
The molecular formula C6H12O6 represents glucose, a simple sugar that is a fundamental component of many carbohydrates. To determine whether glucose is polar or nonpolar, we need to examine its molecular structure and the nature of its bonds.
Glucose is composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. Its structure can be represented in a few different ways, but one of the most common is the chair conformation of its cyclic form. In this structure, five of the carbon atoms and one oxygen atom form a ring, with the sixth carbon atom attached to the ring through an oxygen atom, forming a primary alcohol group (-CH2OH) and several secondary alcohol groups (-CHOH-) around the ring.
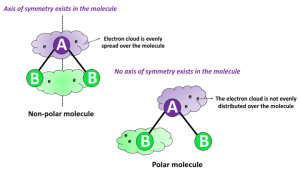
The polarity of a molecule is largely determined by the difference in electronegativity between atoms in a covalent bond and the shape of the molecule. Electronegativity is a measure of an atom’s ability to attract electrons in a covalent bond. Oxygen is more electronegative than carbon and hydrogen, which means it tends to pull electrons towards itself in a covalent bond.
In glucose, the bonds between carbon and hydrogen are relatively nonpolar because the electronegativity difference between carbon and hydrogen is small. However, the bonds between carbon and oxygen are polar due to the significant difference in electronegativity between these atoms. Specifically, the C-O (carbon-oxygen) and C=O (carbon-oxygen double bond, though in the case of glucose in its ring form, this is less relevant) bonds are polar, with the oxygen pulling electrons away from the carbon, creating a partial positive charge on the carbon and a partial negative charge on the oxygen.
Despite the presence of these polar bonds, the overall shape of the glucose molecule and the distribution of its polar groups are such that glucose is considered to be moderately polar but not highly polar. The molecule has several hydroxyl (-OH) groups attached to its ring structure, which can form hydrogen bonds with other glucose molecules or with water, making glucose soluble in water. This ability to form hydrogen bonds, which are relatively strong intermolecular forces compared to other types like dipole-dipole or London dispersion forces, contributes to its polar nature.
However, when considering the molecule as a whole, the polar character is distributed in such a way (due to its three-dimensional structure) that it doesn’t have a significant net dipole moment in the direction that would classify it strictly as a polar molecule like water (H2O) or ammonia (NH3). Instead, the distribution of charge across the molecule, facilitated by its several hydroxyl groups, makes it capable of interacting with both polar and nonpolar substances to some extent, though its interactions with polar substances (like water) are particularly strong due to its ability to form hydrogen bonds.
In summary, while glucose contains polar bonds and can engage in polar interactions, especially through hydrogen bonding, its overall molecular structure and distribution of polar groups mean it exhibits characteristics of both polar and nonpolar molecules. For practical purposes, especially in the context of its solubility in water and biological functions, glucose is often treated as a polar molecule due to its hydroxyl groups and ability to engage in significant interactions with water. However, its classification as strictly polar or nonpolar can depend on the context in which it is being considered.
Is glucose soluble in water due to its polar nature?
+Yes, glucose is soluble in water. Its polar hydroxyl (-OH) groups allow it to form hydrogen bonds with water molecules, which facilitates its dissolution in water.
What contributes to the polar character of glucose?
+The presence of polar bonds, particularly the carbon-oxygen bonds, and the distribution of hydroxyl groups around the glucose molecule contribute to its polar character.
Can glucose interact with nonpolar substances?
+While glucose is more soluble in polar solvents like water due to its ability to form hydrogen bonds, it can also interact with nonpolar substances to some extent. However, these interactions are generally weaker than those with polar substances.
In conclusion, glucose exhibits characteristics that align with both polar and nonpolar molecules, but its ability to form hydrogen bonds and its solubility in water are more indicative of a polar molecule. Its unique structure, with multiple hydroxyl groups, allows it to interact strongly with polar substances, making it a vital component of biological systems, particularly in the context of energy storage and metabolism.