Ch2o Shape Revealed: Master Molecular Geometry
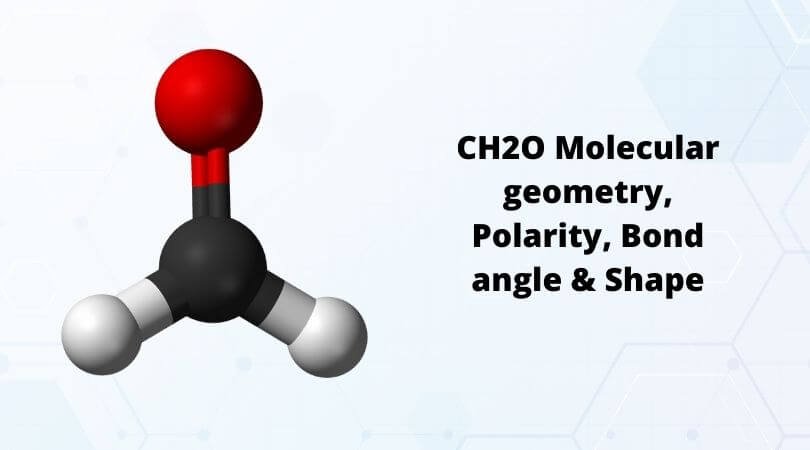
Understanding the molecular geometry of CH2O, commonly known as formaldehyde, is crucial for grasping its chemical properties and behaviors. Formaldehyde is the simplest aldehyde, consisting of a carbon atom bonded to two hydrogen atoms and one oxygen atom through a double bond. The molecule’s shape is determined by the arrangement of its electron groups, which in turn is influenced by the type of bonds and the presence of lone pairs on the central atom.
Electron Group Geometry and Molecular Shape
In the case of CH2O, the central carbon atom is bonded to two hydrogen atoms and one oxygen atom. The carbon-oxygen bond is a double bond, which consists of one sigma (σ) bond and one pi (π) bond. This double bond counts as one electron group due to its directional nature. Thus, around the carbon atom, there are effectively three electron groups: two single bonds (to the hydrogen atoms) and one double bond (to the oxygen atom).
According to VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts the shape of molecules based on the repulsion between electron groups, three electron groups around a central atom arrange themselves in a trigonal planar geometry to maximize the distance between them. However, when considering the actual molecular shape, we must distinguish between electron group geometry (the arrangement of electron groups around the central atom) and molecular shape (the arrangement of atoms in space).
Molecular Shape of CH2O
Given the trigonal planar arrangement of electron groups around the carbon atom in CH2O, and considering that the double bond to oxygen occupies one of these positions, the molecular shape of formaldehyde is also trigonal planar. The planarity of the molecule is due to the sp2 hybridization of the carbon atom, which results from the need to form a double bond with oxygen and single bonds with the two hydrogen atoms. This hybridization leads to a flat, two-dimensional arrangement of the atoms.
Implications of Molecular Geometry
The molecular geometry of CH2O has significant implications for its physical and chemical properties. For instance, the polarity of the carbon-oxygen double bond contributes to the molecule’s dipole moment, making formaldehyde a polar molecule. This polarity affects its solubility, boiling point, and reactivity, particularly in nucleophilic addition reactions where the electrophilic carbon atom of the carbonyl group is attacked by nucleophiles.
Practical Applications and Relevance
Understanding the molecular geometry of CH2O is not merely an academic exercise; it has practical implications in various fields. In organic chemistry, knowledge of formaldehyde’s molecular shape is essential for predicting its reactivity and for designing synthetic routes to more complex molecules. In environmental science, the molecular geometry of formaldehyde influences its atmospheric chemistry, including its participation in photochemical smog formation. In biology, formaldehyde’s reactivity, partly dictated by its molecular shape, is relevant to its use as a disinfectant and preservative.
Theoretical Foundations
Theoretical models, such as molecular orbital theory and density functional theory, provide a deeper understanding of the electronic structure and molecular geometry of CH2O. These theories help in understanding the distribution of electrons, the strength of bonds, and the reactivity of the molecule. Computational chemistry methods based on these theories can predict the molecular geometry, vibrational spectra, and thermochemical properties of formaldehyde, complementing experimental findings.
Experimental Evidence
Experimental techniques such as infrared spectroscopy, microwave spectroscopy, and electron diffraction have been used to determine the molecular geometry of CH2O. These methods provide direct evidence for the trigonal planar arrangement of atoms in the molecule. The experimental data are in good agreement with the predictions based on VSEPR theory and molecular orbital calculations, validating our understanding of the molecular geometry of formaldehyde.
Conclusion
In conclusion, the molecular geometry of CH2O, characterized by a trigonal planar shape, is a critical aspect of its chemical properties and reactivity. Understanding this geometry, through both theoretical models and experimental evidence, is essential for a comprehensive appreciation of formaldehyde’s role in chemistry and its applications across various disciplines. The precise arrangement of atoms in space influences the molecule’s physical properties, its interaction with other molecules, and its participation in chemical reactions, underscoring the importance of molecular geometry in the study of chemistry.
What is the molecular geometry of formaldehyde (CH2O)?
+The molecular geometry of formaldehyde is trigonal planar, due to the sp2 hybridization of the central carbon atom which forms a double bond with oxygen and single bonds with two hydrogen atoms.
Why is the molecular geometry of CH2O important?
+The molecular geometry of CH2O is crucial for understanding its physical and chemical properties, including polarity, solubility, boiling point, and reactivity, especially in nucleophilic addition reactions.
How does the molecular geometry of formaldehyde influence its reactivity?
+The trigonal planar geometry of formaldehyde, with its electrophilic carbon atom in the carbonyl group, makes it highly reactive towards nucleophiles, a key aspect of its participation in various organic reactions.



