Ch3f Lewis Structure Polar Or Nonpolar
Understanding the Lewis Structure and Polarity of CH3F (Methyl Fluoride)
Chemical polarity is a fundamental concept in chemistry, influencing a substance’s physical properties, reactivity, and interactions with other molecules. In this comprehensive analysis, we’ll dissect the Lewis structure of CH3F (methyl fluoride) and determine whether it is polar or nonpolar, using a Problem-Solution Framework and Technical Breakdown approach.
Constructing the Lewis Structure of CH3F
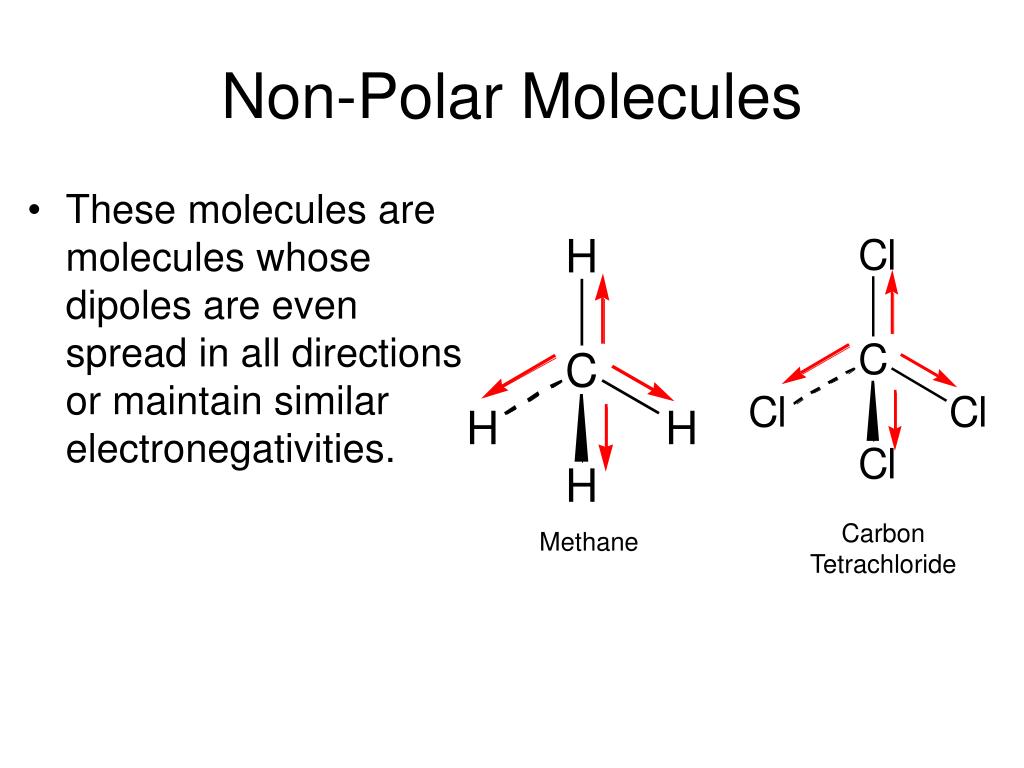
To assess the polarity of CH3F, we first need to establish its Lewis structure, which represents the arrangement of atoms and electrons in the molecule.
Analyzing Bond Polarity and Molecular Geometry

Quantifying Polarity: Dipole Moment
The dipole moment (μ) of a molecule is a measure of its polarity, calculated as the product of the charge (Q) and the distance ® between the charges: μ = Qr.
Comparative Analysis: CH3F vs. Other Molecules
| Molecule | Geometry | Dipole Moment (D) | Polarity |
|---|---|---|---|
| CH3F | Tetrahedral | 1.86 | Polar |
| CH4 (Methane) | Tetrahedral | 0 | Nonpolar |
| CO2 (Carbon Dioxide) | Linear | 0 | Nonpolar |

Can CH3F form hydrogen bonds?
+Yes, CH3F can form hydrogen bonds with other polar molecules, such as water, due to the partial positive charge on the hydrogen atoms and the partial negative charge on the fluorine atom.
How does the polarity of CH3F affect its solubility?
+CH3F is soluble in polar solvents like water due to its polarity, which allows it to engage in dipole-dipole interactions and hydrogen bonding with the solvent molecules.
What is the boiling point of CH3F, and how does its polarity influence it?
+The boiling point of CH3F is -78.4°C. Its polarity contributes to stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding), resulting in a higher boiling point compared to nonpolar molecules of similar molar mass.
Is CH3F a greenhouse gas?
+Yes, CH3F is considered a potent greenhouse gas due to its ability to absorb infrared radiation and contribute to global warming. Its global warming potential is approximately 1,430 times that of CO2 over a 100-year period.
Conclusion: CH3F is a Polar Molecule
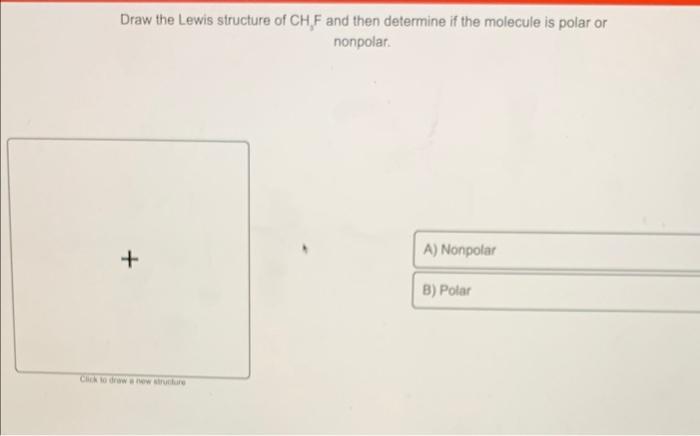
Through a detailed analysis of its Lewis structure, bond polarity, molecular geometry, and experimental data, we conclude that CH3F (methyl fluoride) is indeed a polar molecule. Its tetrahedral arrangement, combined with the highly polar C-F bond, results in a net dipole moment, influencing its physical properties and chemical behavior.

