Ch4 Covalent Or Ionic
The distinction between covalent and ionic bonds is fundamental in understanding the nature of chemical compounds. This dichotomy is crucial because the type of bond that forms between atoms significantly influences the properties of the resulting compound, including its physical state, solubility, melting point, and reactivity.
To delve into this topic, it’s essential to start with the basics of what constitutes a chemical bond. A chemical bond is an attraction between atoms that holds them together in a chemical compound. This attraction is due to the sharing or exchange of electrons between atoms. The primary difference between covalent and ionic bonds lies in how electrons are distributed or exchanged between the atoms involved.
Covalent Bonds
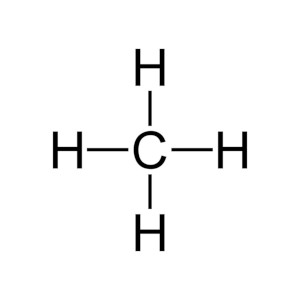
Covalent bonds are formed when two or more atoms share one or more pairs of electrons to achieve a more stable electron configuration, typically resulting in a full outer shell of electrons. This sharing of electrons leads to an attraction between the atoms, holding them together in a molecule. The key characteristic of covalent bonds is that the electrons are not transferred from one atom to another but are shared in such a way that both atoms can consider the shared electrons as part of their outer shell.
Covalent bonds can be further classified into polar covalent bonds and nonpolar covalent bonds. Nonpolar covalent bonds are formed when the electrons are shared equally between the two atoms, resulting in no net dipole moment. Polar covalent bonds, on the other hand, occur when the electrons are shared unequally due to the difference in electronegativity between the atoms, leading to a dipole moment.
Ionic Bonds
Ionic bonds are formed when one or more electrons are transferred between atoms, resulting in the formation of ions with opposite charges. The electrostatic attraction between the positively charged cation and the negatively charged anion holds them together in an ionic compound. This type of bond typically occurs between a metal and a nonmetal, where the metal loses electrons to form a cation, and the nonmetal gains electrons to form an anion.
The characteristics of ionic compounds are often distinctly different from those of covalent compounds. For instance, ionic compounds tend to be solids at room temperature, have high melting and boiling points, and are usually soluble in water. The ionic bond’s nature means that ionic compounds can conduct electricity when dissolved in water or melted, which is not typically the case for covalent compounds.
Comparison of Covalent and Ionic Bonds
Understanding the differences between covalent and ionic bonds is crucial for predicting the properties and behavior of chemical compounds. Here are some key points of comparison:
- Formation: Covalent bonds are formed by the sharing of electrons, while ionic bonds are formed by the transfer of electrons.
- Physical State: At room temperature, covalent compounds can be solids, liquids, or gases, depending on the molecule’s complexity and the strength of the intermolecular forces. Ionic compounds are typically solids.
- Solubility: Covalent compounds are generally less soluble in water compared to ionic compounds, which are often highly soluble due to their ability to dissociate into ions in aqueous solution.
- Melting and Boiling Points: Ionic compounds have higher melting and boiling points than covalent compounds because of the strong electrostatic attractions between ions.
- Conductivity: Ionic compounds can conduct electricity when dissolved in water or melted, whereas most covalent compounds do not conduct electricity under these conditions.
Real-World Applications and Implications
The distinction between covalent and ionic bonds has significant implications for various fields, including materials science, pharmaceuticals, and environmental science. For instance, the strength and type of bonding in materials determine their suitability for different applications. In pharmaceuticals, understanding the bonding nature helps in drug design and interaction with biological molecules. In environmental science, recognizing the bonding types aids in predicting the behavior and fate of pollutants in the environment.
Future Trends and Research Directions
Future research directions may include exploring new materials with unique bonding characteristics for advanced applications, such as supercapacitors, batteries, and nanotechnology. Additionally, there is ongoing interest in understanding and manipulating the bonding in biomolecules for medical and biotechnological applications. The development of computational methods and machine learning algorithms to predict and design materials with specific bonding properties is also an area of active research.
FAQ Section
What is the primary difference between covalent and ionic bonds?
+The primary difference between covalent and ionic bonds lies in how electrons are distributed or exchanged between the atoms involved. Covalent bonds involve the sharing of electrons, while ionic bonds involve the transfer of electrons, resulting in the formation of ions with opposite charges.
Which type of bond typically occurs between a metal and a nonmetal?
+An ionic bond typically occurs between a metal and a nonmetal, where the metal loses electrons to form a cation, and the nonmetal gains electrons to form an anion.
Why are ionic compounds generally more soluble in water than covalent compounds?
+Ionic compounds are generally more soluble in water because they can dissociate into ions in aqueous solution, which allows them to interact more effectively with water molecules.
What determines the conductivity of a compound?
+The conductivity of a compound is largely determined by its ability to conduct electricity, which in turn is influenced by the type of bonding. Ionic compounds can conduct electricity when dissolved in water or melted due to the movement of ions.
How does understanding the difference between covalent and ionic bonds impact real-world applications?
+Understanding the difference between covalent and ionic bonds has significant implications for various fields, including materials science, pharmaceuticals, and environmental science, as it influences the properties and behavior of chemical compounds.