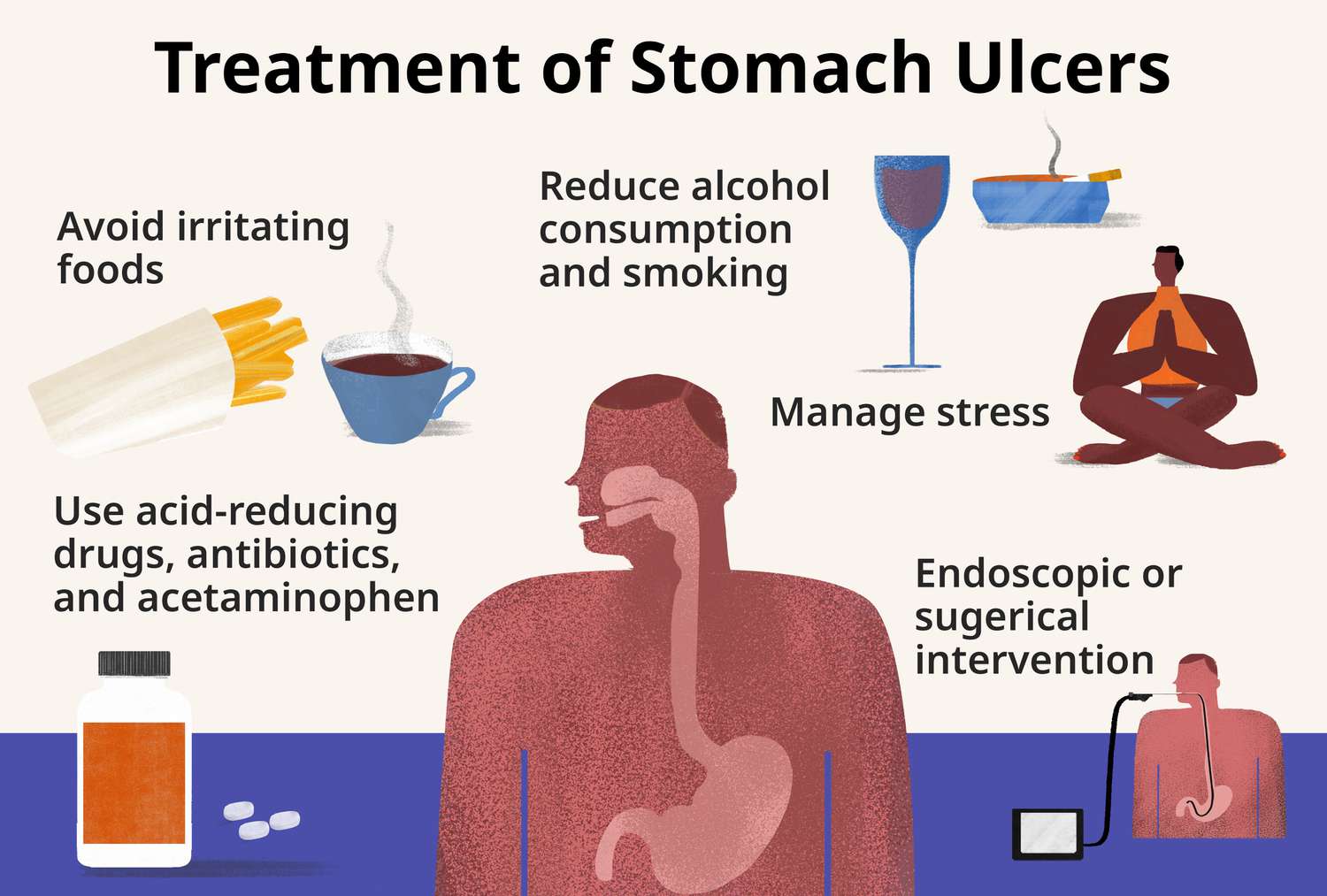
Chemistry Product: Learn Formulas And Reactions
The realm of chemistry is a fascinating domain that underlies the very fabric of our universe, governing the interactions between substances and energy. At its core, chemistry is about understanding the formulas and reactions that define how different elements and compounds interact with each other. This understanding is not just abstract knowledge; it has practical applications in everything from the development of new pharmaceuticals to the creation of more efficient energy storage devices.
Foundations of Chemistry: Understanding Formulas
Chemical formulas are symbolic representations of the composition of molecules, indicating the types and proportions of atoms present in a molecule. For instance, water is represented by the formula H2O, signifying that a molecule of water consists of two hydrogen atoms bonded to a single oxygen atom. Understanding chemical formulas is crucial because it allows chemists to predict properties of substances, such as their reactivity, boiling and melting points, and solubility in various solvents.
One of the foundational concepts in understanding chemical formulas is the concept of stoichiometry. Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. It’s based on the law of conservation of mass, which states that matter cannot be created or destroyed in a chemical reaction. Thus, the number of atoms for each element must be the same on both the reactant and product sides of a chemical equation. This principle is fundamental in calculating the amounts of substances required or produced in reactions, a critical aspect of both laboratory experimentation and industrial chemical production.
Chemical Reactions: Introduction and Types
Chemical reactions involve the transformation of one or more substances into another substance or substances. These reactions are characterized by the breaking and forming of chemical bonds between atoms, which can result in changes to the chemical properties of the substances involved. There are several types of chemical reactions, including synthesis (combination) reactions, decomposition reactions, replacement (substitution) reactions, and combustion reactions, among others.
Synthesis Reactions: These are reactions where two or more substances combine to form a new compound. An example is the reaction between sodium (Na) and chlorine (Cl2) to form sodium chloride (NaCl), which is table salt.
Decomposition Reactions: In these reactions, a single compound breaks down into two or more simpler substances. For example, water (H2O) can decompose into hydrogen gas (H2) and oxygen gas (O2) when an electric current is passed through it.
Replacement Reactions: These reactions involve one element replacing another element from a compound. A classic example is the reaction between zinc (Zn) and copper sulfate (CuSO4), where zinc displaces copper to form zinc sulfate (ZnSO4) and copper (Cu).
Combustion Reactions: These are reactions where a substance reacts with oxygen, typically producing heat and light. The combustion of methane (CH4), a major component of natural gas, in oxygen (O2) to produce carbon dioxide (CO2) and water (H2O) is a fundamental reaction in both industrial processes and everyday life.
Practical Applications of Chemical Reactions
Chemical reactions have a myriad of applications across various sectors. In the pharmaceutical industry, understanding chemical reactions is crucial for the development of new drugs and the improvement of existing ones. The synthesis of complex molecules involves a series of carefully controlled chemical reactions, each step requiring a deep understanding of reaction conditions, catalysts, and reagents.
In the field of materials science, chemical reactions are used to create new materials with unique properties, such as superconductors, nanomaterials, and polymers. These materials have applications ranging from consumer electronics to aerospace engineering.
Moreover, chemical reactions play a central role in environmental science and technology. Understanding the chemical reactions that occur in the atmosphere, water, and soil is essential for addressing pollution and climate change. Technologies like catalytic converters in vehicles and scrubbers in power plants rely on chemical reactions to reduce harmful emissions.
Conclusion
In conclusion, the study of chemical formulas and reactions is the cornerstone of chemistry. It not only provides a fundamental understanding of the molecular world but also has vast practical implications. From the development of life-saving medications to the creation of sustainable energy solutions, chemical knowledge is pivotal. As we continue to face global challenges such as climate change, energy security, and public health, the importance of advancing our understanding of chemical reactions and their applications will only continue to grow.
Frequently Asked Questions
What is the significance of understanding chemical formulas?
+Understanding chemical formulas is crucial because it allows for the prediction of properties of substances, such as their reactivity, boiling and melting points, and solubility. This knowledge is essential in laboratory experimentation, industrial chemical production, and the development of new materials and drugs.
<div class="faq-item">
<div class="faq-question">
<h3>How do chemical reactions impact our daily lives?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Chemical reactions have a profound impact on our daily lives, from the food we eat to the air we breathe. They are involved in the production of virtually every consumer product, pharmaceutical, and energy source. Additionally, chemical reactions in the environment, such as those in the atmosphere and waterways, directly influence our health and the sustainability of our planet.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>What role do chemical reactions play in addressing global challenges?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Chemical reactions play a critical role in addressing global challenges such as climate change, energy security, and public health. Through the development of sustainable energy technologies, more efficient industrial processes, and innovative solutions for pollution mitigation, chemistry offers key strategies for mitigating these challenges. Furthermore, chemical research into new materials and reactions can lead to breakthroughs in fields like renewable energy, advanced water treatment, and disease prevention.</p>
</div>
</div>
<div class="faq-item">
<div class="faq-question">
<h3>How can learning about chemical formulas and reactions benefit a non-chemistry professional?</h3>
<span class="faq-toggle">+</span>
</div>
<div class="faq-answer">
<p>Learning about chemical formulas and reactions can benefit anyone by providing a deeper understanding of the world around them. It can enlighten individuals about the principles behind many common phenomena and products, enhancing their ability to make informed decisions regarding health, environment, and technology. Moreover, it fosters critical thinking and analytical skills, which are valuable in any profession.</p>
</div>
</div>
</div>
By embracing the study of chemical formulas and reactions, we not only delve into the intricacies of the molecular world but also open up avenues for innovation and problem-solving that can transform our future. Whether you are a professional in the field or simply an inquisitive learner, the realm of chemistry offers a wealth of knowledge and discovery waiting to be uncovered.