Conjugate Base Of Ammonia
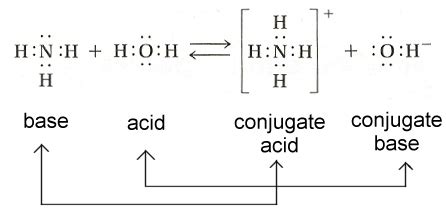
The conjugate base of ammonia (NH3) is the amide ion (NH2-). This is formed when ammonia accepts a proton (H+), resulting in the equilibrium reaction:
NH3 + H2O ⇌ NH4+ + OH-
However, if we consider the removal of a proton from ammonia, which is a less common reaction due to ammonia’s basic nature, the conjugate base would indeed be the amide ion:
NH3 ⇌ NH2- + H+
The amide ion is a strong base and is not typically found in aqueous solutions due to its high reactivity with water, forming ammonia:
NH2- + H2O → NH3 + OH-
Understanding the conjugate base of ammonia is crucial in various chemical reactions, particularly in organic chemistry, where the basic properties of ammonia and its conjugate base play significant roles in synthesis and catalysis.
Properties of Amide Ion
- Basicity: The amide ion is an extremely strong base. Its basicity is reflected in its ability to abstract protons from a wide range of substances, including water, making it highly reactive in aqueous solutions.
- Stability: Due to its high reactivity, the amide ion is not stable in aqueous solution, immediately reacting with water to form ammonia and hydroxide ions.
- Reactivity: The amide ion’s reactivity makes it a useful intermediate in organic synthesis. It can participate in various reactions, such as nucleophilic substitutions and eliminations, due to its strong nucleophilic character.
Role in Organic Chemistry
In organic chemistry, the generation and use of the amide ion (or its equivalents) are crucial for performing certain types of reactions, such as:
- Nucleophilic Substitutions: The amide ion can act as a strong nucleophile, attacking electrophilic centers in molecules, such as alkyl halides, to form new bonds.
- Elimination Reactions: Amide ions can abstract protons from molecules, leading to the formation of new bonds and the elimination of leaving groups, resulting in the creation of alkenes or alkynes.
Generation of Amide Ion
The generation of the amide ion in the laboratory often involves the reaction of ammonia or an amine with a strong base. For example:
2NH3 + 2Na → 2NH2- + H2
This reaction involves the use of sodium (or another highly electropositive metal) to remove protons from ammonia, producing the amide ion and hydrogen gas.
Conclusion
The conjugate base of ammonia, the amide ion, is a fundamental concept in chemistry, showcasing the complex behavior of simple molecules in different chemical environments. Its extreme basicity and reactivity make it a valuable but challenging species to work with, particularly in the context of organic synthesis and catalysis.
Understanding the properties and reactivity of the amide ion provides valuable insights into the design of new synthetic methodologies and the optimization of existing chemical processes.
Practical Applications
- Organic Synthesis: The use of amide ions or their equivalents in organic synthesis has led to the development of numerous methodologies for the formation of C-C and C-N bonds.
- Catalysis: The basic properties of the amide ion make it a useful species in catalytic reactions, particularly in those requiring strong bases.
Future Directions
The study of the amide ion and its equivalents continues to be an active area of research, with scientists exploring new methods for its generation and application in synthesis and catalysis. The development of more stable and manageable equivalents of the amide ion could open up new avenues for chemical transformations, contributing to advancements in fields such as pharmaceutical synthesis and materials science.
Formation and Reaction of Amide Ion
- Generation of the amide ion through the reaction of ammonia with a strong base.
- Reaction of the amide ion with water, resulting in the formation of ammonia and hydroxide ions.
- Utilization of the amide ion in organic synthesis, including nucleophilic substitutions and elimination reactions.
Frequently Asked Questions
What is the conjugate base of ammonia?
+The conjugate base of ammonia is the amide ion (NH2-), formed by the removal of a proton from ammonia.
Is the amide ion stable in aqueous solutions?
+No, the amide ion is highly reactive with water, immediately forming ammonia and hydroxide ions, making it unstable in aqueous solutions.
What are the practical applications of the amide ion?
+The amide ion has practical applications in organic synthesis, particularly in the formation of new bonds through nucleophilic substitutions and eliminations, and in catalysis.
