Dalton In G Mol: Understand Molar Mass Easily
The concept of molar mass is fundamental in chemistry, serving as a bridge between the microscopic world of atoms and molecules and the macroscopic world of measurable quantities. It’s defined as the mass of one mole of a substance, with the mole being the unit of measurement that represents 6.022 x 10^23 particles (atoms or molecules). Understanding molar mass is crucial for calculating the quantities of reactants and products in chemical reactions, as well as for determining the composition of mixtures.
Historical Development of Molar MassConcept
The history of the molar mass concept is closely tied to the development of modern chemistry. Early chemists, such as Antoine Lavoisier and Joseph Proust, laid the groundwork by discovering the law of conservation of mass and the law of definite proportions, respectively. However, it wasn’t until the work of Amedeo Avogadro in the early 19th century that the concept of the mole began to take shape. Avogadro’s hypothesis stated that equal volumes of gases at the same temperature and pressure contain an equal number of molecules, which eventually led to the definition of the mole and the calculation of molar masses.
Calculating Molar Mass
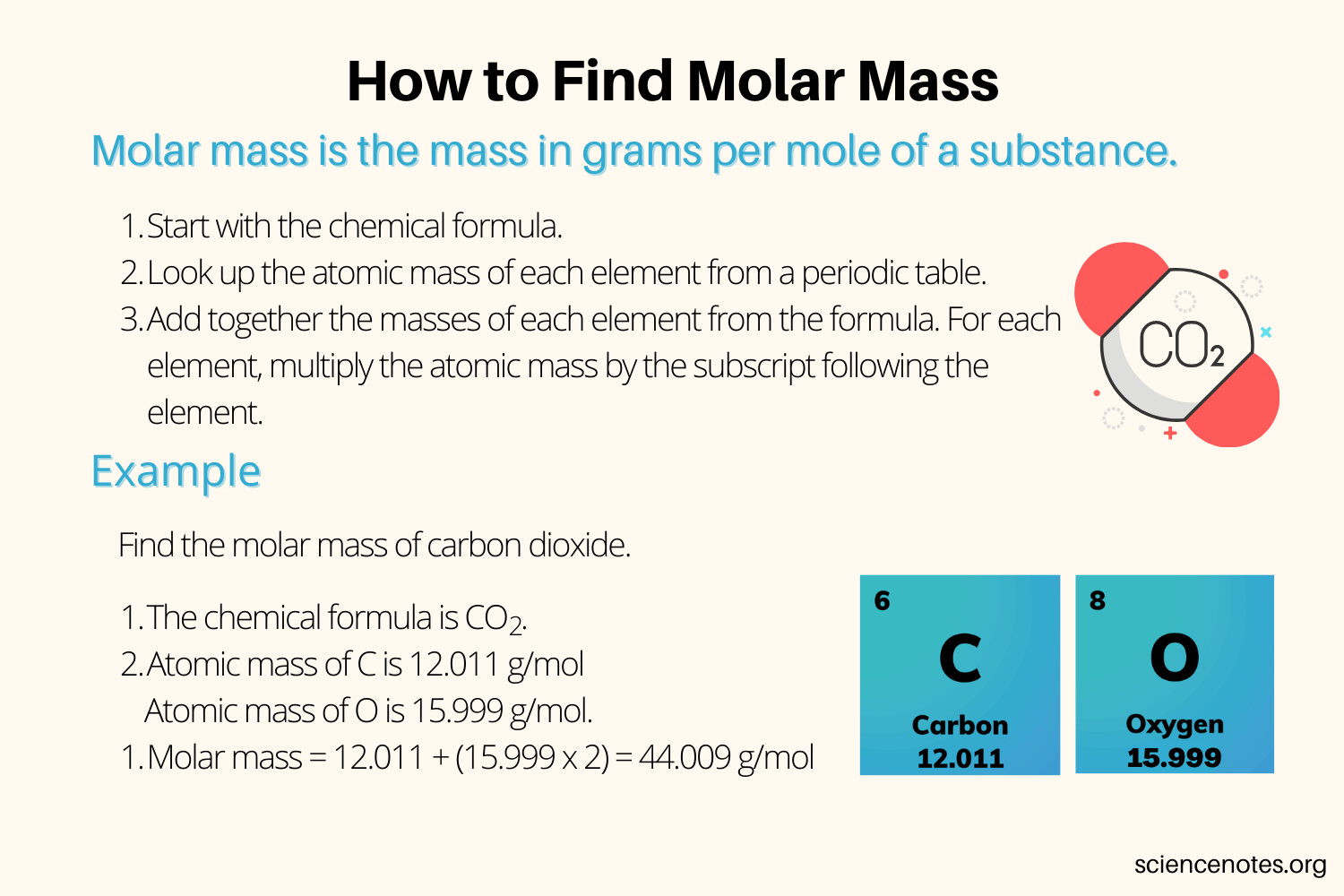
Calculating the molar mass of a substance involves summing the atomic masses of all the atoms in a molecule of the substance. Atomic masses are found on the periodic table and represent the average mass of an atom of an element, taking into account its naturally occurring isotopes. For example, to calculate the molar mass of water (H2O), one would add the atomic masses of two hydrogen atoms to the atomic mass of one oxygen atom.
- Atomic mass of hydrogen (H) = 1.00794 g/mol
- Atomic mass of oxygen (O) = 15.9994 g/mol
Molar mass of water = (2 * atomic mass of H) + atomic mass of O = (2 * 1.00794 g/mol) + 15.9994 g/mol = 2.01588 g/mol + 15.9994 g/mol = 18.01528 g/mol
Thus, the molar mass of water is approximately 18.015 g/mol.
Importance of Molar Mass in Chemistry
Molar mass plays a pivotal role in various chemical calculations, including:
Determination of Empirical and Molecular Formulas: By knowing the molar mass of a compound and the mass percentages of its constituent elements, one can determine its empirical and molecular formulas.
Reaction Stoichiometry: Molar masses are essential for calculating the amounts of substances involved in chemical reactions, allowing chemists to predict how much product will be formed from given amounts of reactants.
Concentration of Solutions: The molar mass is used to calculate the molarity of a solution, which is the number of moles of solute per liter of solution.
Gas Laws: For gases, molar mass is crucial in applying the ideal gas law, PV = nRT, where n is the number of moles of gas.
Practical Applications of Molar Mass
Beyond theoretical calculations, the concept of molar mass has numerous practical applications in fields such as pharmacy, where it’s used to prepare solutions and calculate drug dosages; in the food industry, for nutritional labeling and quality control; and in environmental science, for assessing the concentration of pollutants in air and water.
Challenges and Limitations
While the concept of molar mass is well-established, challenges can arise in its application, especially when dealing with mixtures of substances or compounds with complex molecular structures. Additionally, the molar mass of a substance can vary slightly depending on the isotopic composition of the sample, though this variation is usually negligible for most practical purposes.
Future Developments and Emerging Trends
As analytical techniques continue to improve, allowing for more precise measurements of atomic and molecular masses, our understanding of molar mass and its applications is likely to evolve. Advances in mass spectrometry, for example, enable the determination of exact molecular masses, which is crucial for the identification and characterization of complex biomolecules and synthetic compounds.
Conclusion
In conclusion, molar mass is a foundational concept in chemistry that bridges the gap between the atomic level and the macroscopic world. Its calculation and application are fundamental to understanding chemical reactions, determining the composition of substances, and predicting the behavior of gases. As chemistry continues to advance, the role of molar mass will remain central to both theoretical and practical applications across various disciplines.
What is the significance of Avogadro’s number in calculating molar mass?
+Avogadro’s number (6.022 x 10^23) is crucial because it defines the number of particles (atoms or molecules) in one mole of a substance. This allows for the conversion between the amount of a substance and its mass, which is essential for calculating molar mass.
How does the molar mass of elements and compounds differ?
+The molar mass of an element is the mass of one mole of its atoms, while the molar mass of a compound is the sum of the molar masses of its constituent atoms. For elements, it’s essentially the atomic mass multiplied by the number of atoms in the molecule for diatomic or polyatomic elements like hydrogen (H2) or oxygen (O2), respectively.
What are some common units of molar mass?
+Molar mass is typically expressed in units of grams per mole (g/mol), though for very large molecules like proteins or polymers, it might be more convenient to use kilograms per mole (kg/mol).
