Freezing Temperature Of Water: Know The Exact Point
The freezing temperature of water is a fundamental concept in physics and chemistry, and it’s essential to understand the exact point at which water changes its state from liquid to solid. In this article, we’ll delve into the world of thermodynamics and explore the fascinating process of water freezing.
Introduction to Water Freezing
Water is a unique substance that expands when it freezes, which is why ice floats on top of liquid water. This phenomenon is crucial for many natural processes, including the formation of sea ice, glaciers, and icebergs. The freezing point of water is the temperature at which the liquid changes its state to become a solid, and it’s a critical parameter in various fields, such as chemistry, biology, and engineering.
The Exact Freezing Point of Water
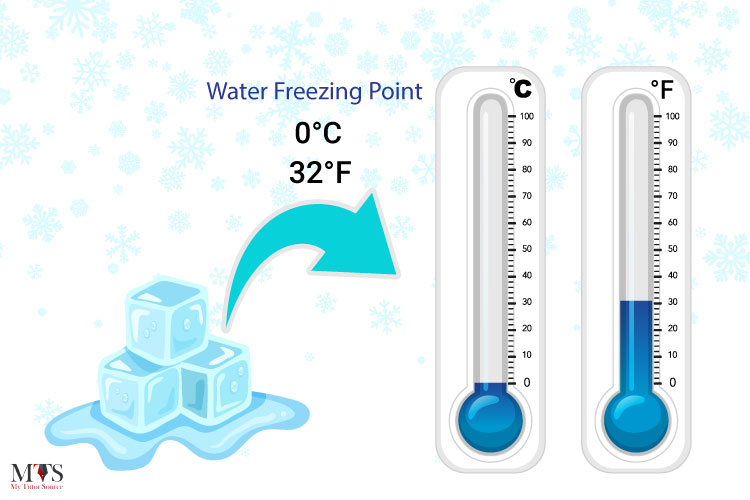
The freezing point of water is 0 degrees Celsius (°C) or 32 degrees Fahrenheit (°F) at standard atmospheric pressure. However, this value can vary slightly depending on the purity of the water and the presence of impurities or dissolved substances. For instance, seawater freezes at a lower temperature than fresh water due to its higher salinity.
The Science Behind Water Freezing
When water cools down, the molecules slow down and come closer together. As the temperature decreases, the molecules start to form a crystal lattice structure, which is the characteristic arrangement of atoms or molecules in a solid. The process of water freezing involves the formation of hydrogen bonds between the water molecules, which are weak electrostatic attractions that hold the molecules together.
Factors Affecting the Freezing Point of Water
Several factors can influence the freezing point of water, including:
- Pressure: The freezing point of water decreases with increasing pressure. This is why water can remain in a liquid state at temperatures below 0°C under high pressure.
- Salinity: The presence of dissolved substances, such as salt or sugar, can lower the freezing point of water.
- Dissolved gases: The presence of dissolved gases, such as air or carbon dioxide, can affect the freezing point of water.
- Surface tension: The surface tension of water can influence the freezing point, especially in small droplets or thin films.
Practical Applications of Water Freezing
The freezing point of water has numerous practical applications in various fields, including:
- Cryogenics: The study of the behavior of materials at extremely low temperatures, which is crucial for applications such as superconductivity and cryopreservation.
- Food preservation: The freezing point of water is essential for preserving food, as it allows for the storage of perishable items at low temperatures.
- Climate modeling: The freezing point of water is a critical parameter in climate modeling, as it affects the formation of sea ice and glaciers, which in turn influence global climate patterns.
The freezing point of water is not just a simple physical constant; it's a complex phenomenon that involves the interplay of various factors, including pressure, salinity, and surface tension. Understanding the exact point at which water freezes is crucial for many practical applications, from food preservation to climate modeling.
Comparison of Freezing Points
Here’s a comparison of the freezing points of different substances:
| Substance | Freezing Point (°C) |
|---|---|
| Water | 0 |
| Seawater | -1.8 |
| Ethanol | -114 |
| Methanol | -98 |
| Glycerol | -18 |

What is the freezing point of water at high pressure?
+The freezing point of water decreases with increasing pressure. At a pressure of 1000 bar, the freezing point of water is around -20°C.
How does the presence of impurities affect the freezing point of water?
+The presence of impurities, such as salt or sugar, can lower the freezing point of water. This is known as freezing-point depression.
What is the significance of the freezing point of water in climate modeling?
+The freezing point of water is a critical parameter in climate modeling, as it affects the formation of sea ice and glaciers, which in turn influence global climate patterns.
Conclusion
In conclusion, the freezing temperature of water is a complex phenomenon that involves the interplay of various factors, including pressure, salinity, and surface tension. Understanding the exact point at which water freezes is crucial for many practical applications, from food preservation to climate modeling. By exploring the science behind water freezing and its practical applications, we can gain a deeper appreciation for the importance of this fundamental physical constant.
