How Does Ir Spectroscopy Identify Aldehydes? Fast Results
The realm of infrared spectroscopy (IR) is a powerful tool for identifying various functional groups in organic molecules, including aldehydes. Aldehydes, characterized by the presence of a carbonyl group (C=O) bonded to at least one hydrogen atom, exhibit distinct IR absorption patterns that can be used for their identification. This article delves into the specifics of how IR spectroscopy identifies aldehydes, providing a comprehensive overview of the process and its interpretations.
Understanding IR Spectroscopy
Before diving into the specifics of identifying aldehydes, it’s essential to understand the basics of IR spectroscopy. IR spectroscopy involves exposing a molecule to infrared radiation and measuring the absorption of radiation by the molecule’s bonds. Different bonds absorb radiation at specific wavelengths, resulting in a unique spectrum for each molecule. This spectrum serves as a fingerprint that can be used to identify the molecule.
Aldehyde Functional Group
Aldehydes have a carbonyl group (C=O) directly attached to a hydrogen atom, which is a key feature distinguishing them from other carbonyl-containing compounds like ketones. This carbonyl group is responsible for several characteristic absorptions in the IR spectrum.
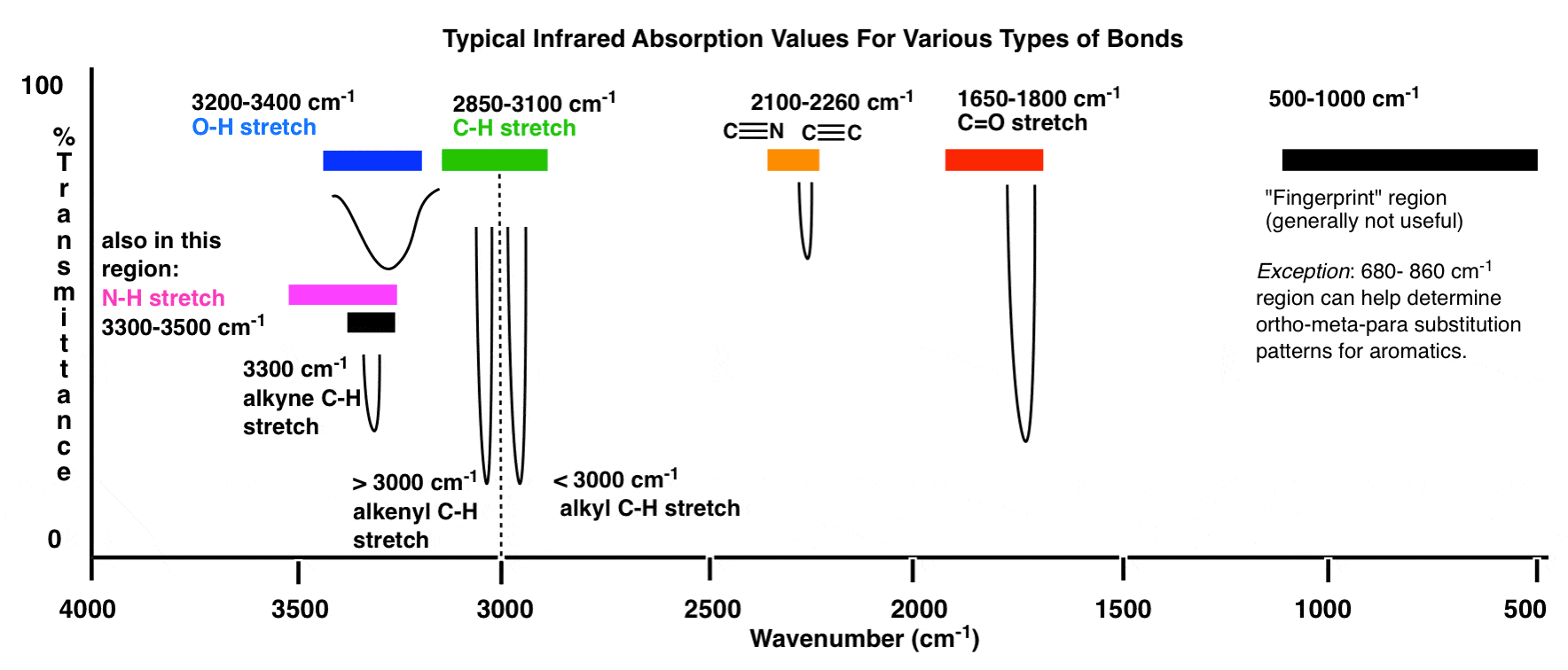
Characteristic IR Absorptions of Aldehydes
The IR spectrum of an aldehyde typically exhibits several prominent absorptions that are diagnostic for the aldehyde functional group. These include:
- C=O Stretch: The most notable absorption for aldehydes is the carbonyl stretch, which usually appears between 1720-1740 cm^-1. This absorption is strong and sharp, due to the polar nature of the C=O bond.
- C-H Stretch: Aldehydes also show a characteristic C-H stretch associated with the aldehyde hydrogen. This typically appears as a weak to medium absorption around 2700-2800 cm^-1 (often referred to as the “aldehyde C-H stretch”). This absorption is usually less intense than other C-H stretches in the molecule.
- C-H Bending: In addition to the stretches, aldehydes can exhibit a C-H bending vibration, which may appear as a weak absorption in the region around 1360-1400 cm^-1.
Interpreting IR Spectra for Aldehyde Identification
When interpreting an IR spectrum to identify an aldehyde, look for the following:
- Presence of a Strong C=O Absorption: A strong absorption in the 1720-1740 cm^-1 range suggests the presence of a carbonyl group.
- Aldehyde C-H Stretch: The presence of a weak to medium absorption in the 2700-2800 cm^-1 range is indicative of the aldehyde hydrogen.
- Contextual Analysis: Consider the overall molecular context. Aldehydes will not exhibit absorptions characteristic of other functional groups unless they are part of a more complex molecule.
Fast Results and Practical Applications
IR spectroscopy provides rapid results, making it an invaluable tool in organic chemistry for the quick identification of functional groups, including aldehydes. The speed and specificity of IR spectroscopy are particularly beneficial in:
- Qualitative Analysis: Quickly determining the presence of aldehydes in a sample.
- Monitoring Reactions: IR spectroscopy can be used to monitor the progress of reactions involving aldehydes, such as oxidations or reductions.
- Structural Elucidation: In conjunction with other spectroscopic methods (like NMR), IR spectroscopy helps in the complete structural elucidation of organic compounds.
Conclusion
IR spectroscopy is a pivotal analytical technique for identifying aldehydes based on their characteristic absorptions. By understanding and recognizing these absorptions, chemists can quickly and accurately identify aldehydes within complex molecules. The combination of speed, specificity, and the non-destructive nature of IR spectroscopy makes it an indispensable tool in modern organic chemistry research and analysis.
FAQs
What is the primary absorption range for the C=O stretch in aldehydes?
+The primary absorption range for the C=O stretch in aldehydes is between 1720-1740 cm^-1.
How does the IR spectrum distinguish aldehydes from ketones?
+Aldehydes are distinguished from ketones by the presence of the aldehyde C-H stretch (around 2700-2800 cm^-1) and specific patterns in the C=O stretch region. Ketones typically exhibit a stronger C=O absorption at slightly lower wavenumbers compared to aldehydes.
What are the practical applications of identifying aldehydes using IR spectroscopy?
+Practical applications include qualitative analysis, monitoring the progress of chemical reactions involving aldehydes, and structural elucidation of organic compounds. IR spectroscopy's speed and non-destructive nature make it particularly useful for these applications.
In conclusion, IR spectroscopy offers a powerful and rapid method for identifying aldehydes through their characteristic IR absorptions, providing invaluable insights in organic chemistry research and analysis.

