How Does Xenon Electron Config Work? Simple Explanation

To grasp how xenon’s electron configuration works, let’s first understand what electron configuration is. Electron configuration is the arrangement of electrons in an atom, which dictates its chemical properties. It’s essentially a map of where the electrons are located around the nucleus of an atom.
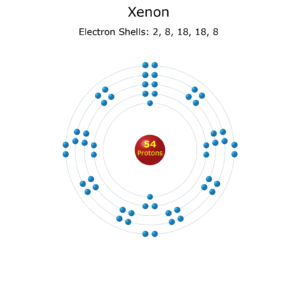
Xenon is a noble gas, situated in the far right column of the periodic table. Noble gases are known for their full outer electron shells, which makes them particularly stable and unreactive. The atomic number of xenon is 54, meaning it has 54 electrons.
The electron configuration of xenon can be broken down as follows: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶. This configuration tells us how the electrons are distributed across different energy levels or shells around the xenon nucleus.
Let’s break this down simply: - 1s²: This means the first energy level (or shell) has 2 electrons in the s-orbital. - 2s² 2p⁶: The second energy level has 2 electrons in the s-orbital and 6 electrons in the p-orbitals. - 3s² 3p⁶: The third energy level has 2 electrons in the s-orbital and 6 electrons in the p-orbitals. - 4s² 3d¹⁰ 4p⁶: The fourth energy level has 2 electrons in the s-orbital, 10 electrons in the d-orbitals of the third energy level (since the d-orbitals are lower in energy than the 4s), and 6 electrons in the p-orbitals. - 5s² 4d¹⁰ 5p⁶: Finally, the fifth energy level has 2 electrons in the s-orbital, 10 electrons in the d-orbitals of the fourth energy level, and 6 electrons in the p-orbitals.
This configuration signifies that xenon’s outermost energy level is completely filled, which is why it’s chemically inert under normal conditions. The full outer shell configuration (ns² np⁶) for noble gases like xenon explains their stability and lack of reactivity, as they don’t readily gain, lose, or share electrons to form compounds.
Understanding xenon’s electron configuration not only helps in grasping the fundamental principles of chemistry but also sheds light on the unique properties of noble gases and their significance in various applications, from lighting to medical uses.
Historical Evolution of Understanding Electron Configuration
The concept of electron configuration has evolved significantly over the years, from the early models of the atom by J.J. Thomson and Ernest Rutherford, to the more sophisticated understanding provided by the quantum mechanics principles developed by Niels Bohr, Louis de Broglie, and Erwin Schrödinger. Today, our understanding of electron configuration is crucial for explaining the periodic properties of elements and predicting their chemical behavior.
Technical Breakdown: Orbitals and Energy Levels

- s-orbitals are spherical and can hold up to 2 electrons.
- p-orbitals are dumbbell-shaped and can hold up to 6 electrons.
- d-orbitals are four-leaf clover-shaped and can hold up to 10 electrons.
- f-orbitals are more complex and can hold up to 14 electrons.
Each energy level can hold a specific number of electrons, and understanding how these electrons are arranged in orbitals around the nucleus is key to predicting chemical properties.
Step-by-Step Guide to Writing Electron Configuration:
- Determine the total number of electrons in the atom.
- Fill the electrons into the lowest available energy levels, starting with 1s, then 2s, 2p, 3s, 3p, and so on.
- Follow the Aufbau principle, where electrons occupy the lowest available energy levels.
- Apply the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of quantum numbers.
- Hund's Rule also comes into play for orbitals of equal energy, stating that electrons will occupy each available orbital singly before any pairing occurs.
Natural Language Explanation: Why Xenon is Inert
Xenon’s inertness can be likened to a full house. Imagine each orbital as a room in a house, and each electron as a person. When all the rooms are filled (as in the case of xenon’s electron configuration), there’s no space for additional guests (electrons from other atoms). This full house scenario is what makes xenon so stable and unreactive; it doesn’t need to gain, lose, or share electrons to fill its “rooms.”
Conclusion
Xenon’s electron configuration is a fascinating example of how the arrangement of electrons in an atom dictates its chemical properties. Understanding this configuration not only deepens our appreciation for the intricacies of atomic structure but also underlines the significance of noble gases in both theoretical chemistry and practical applications.
What makes xenon so chemically stable?
+Xenon’s chemical stability comes from its full outer electron shell. With its outermost energy level completely filled, xenon doesn’t readily react with other elements to gain, lose, or share electrons.
How does xenon’s electron configuration look?
+Xenon’s electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ 5s² 4d¹⁰ 5p⁶, indicating a full outer shell which contributes to its stability.
What are some applications of xenon due to its inert properties?
+Xenon is used in high-intensity lamps, lasers, and as an anesthetic in medical imaging, particularly in magnetic resonance imaging (MRI), due to its inertness and safety profile.
