How To Identify Carboxylic Acid Ir? Easy Tips
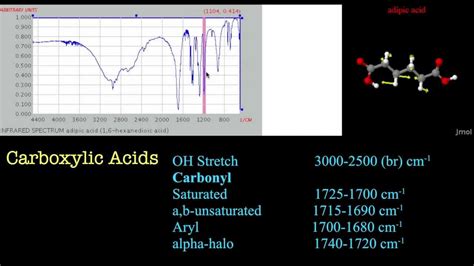
Carboxylic acids are a fundamental class of organic compounds, and identifying them using infrared spectroscopy (IR) is a crucial skill in organic chemistry. IR spectroscopy is a powerful tool for identifying the functional groups present in a molecule, and carboxylic acids have distinct IR absorptions that can be used to identify them. Here’s a step-by-step guide on how to identify carboxylic acids using IR spectroscopy, along with some easy tips to help you master this technique.
Understanding IR Spectroscopy
Before diving into the identification of carboxylic acids, it’s essential to understand the basics of IR spectroscopy. IR spectroscopy works by measuring the absorption of infrared radiation by a molecule. Different functional groups in a molecule absorb infrared radiation at specific wavenumber ranges, resulting in a unique IR spectrum. This spectrum can be used as a fingerprint to identify the molecule.
Identifying Carboxylic Acids
Carboxylic acids have several distinct IR absorptions that can be used to identify them. Here are the key absorptions to look for:
- Broad O-H Stretch: Carboxylic acids typically show a broad O-H stretch absorption between 3600-2400 cm^-1. This absorption is often very broad and can be quite intense.
- C=O Stretch: The C=O stretch absorption in carboxylic acids is usually found between 1720-1680 cm^-1. This absorption is typically sharp and intense.
- C-O Stretch: The C-O stretch absorption in carboxylic acids is usually found between 1320-1210 cm^-1. This absorption is often less intense than the C=O stretch.
- O-H Bend: The O-H bend absorption in carboxylic acids is usually found between 1440-1390 cm^-1. This absorption is often less intense than the C=O stretch.
Easy Tips for Identifying Carboxylic Acids
Here are some easy tips to help you identify carboxylic acids using IR spectroscopy:
- Look for the Broad O-H Stretch: The broad O-H stretch absorption is often the most distinctive feature of a carboxylic acid IR spectrum. If you see a broad absorption between 3600-2400 cm^-1, it’s likely a carboxylic acid.
- Check for the C=O Stretch: The C=O stretch absorption is typically sharp and intense in carboxylic acids. If you see a sharp absorption between 1720-1680 cm^-1, it’s likely a carboxylic acid.
- Use the C-O Stretch and O-H Bend: While the C-O stretch and O-H bend absorptions are often less intense than the C=O stretch, they can still be useful for identifying carboxylic acids. Look for absorptions between 1320-1210 cm^-1 and 1440-1390 cm^-1, respectively.
- Compare with Known Spectra: If you’re unsure about the identity of a carboxylic acid, compare its IR spectrum with known spectra of carboxylic acids. This can help you confirm the identity of the compound.
Common Carboxylic Acids and Their IR Spectra
Here are some common carboxylic acids and their IR spectra:
- Acetic Acid (CH3COOH): Broad O-H stretch at 3600-2400 cm^-1, C=O stretch at 1715 cm^-1, C-O stretch at 1280 cm^-1, O-H bend at 1420 cm^-1.
- Propionic Acid (CH3CH2COOH): Broad O-H stretch at 3600-2400 cm^-1, C=O stretch at 1710 cm^-1, C-O stretch at 1270 cm^-1, O-H bend at 1410 cm^-1.
- Butyric Acid (CH3CH2CH2COOH): Broad O-H stretch at 3600-2400 cm^-1, C=O stretch at 1705 cm^-1, C-O stretch at 1260 cm^-1, O-H bend at 1400 cm^-1.
By following these easy tips and understanding the characteristic IR absorptions of carboxylic acids, you’ll be able to identify these compounds with confidence. Remember to always compare your IR spectra with known spectra and use the characteristic absorptions to confirm the identity of the compound.
FAQ Section
What is the characteristic IR absorption of a carboxylic acid?
+The characteristic IR absorptions of a carboxylic acid include a broad O-H stretch between 3600-2400 cm^-1, a C=O stretch between 1720-1680 cm^-1, a C-O stretch between 1320-1210 cm^-1, and an O-H bend between 1440-1390 cm^-1.
How do I identify a carboxylic acid using IR spectroscopy?
+To identify a carboxylic acid using IR spectroscopy, look for the characteristic IR absorptions, including the broad O-H stretch, C=O stretch, C-O stretch, and O-H bend. Compare your IR spectrum with known spectra of carboxylic acids to confirm the identity of the compound.
What are some common carboxylic acids and their IR spectra?
+Some common carboxylic acids and their IR spectra include acetic acid, propionic acid, and butyric acid. Their IR spectra show characteristic absorptions, including broad O-H stretches, C=O stretches, C-O stretches, and O-H bends.
In conclusion, identifying carboxylic acids using IR spectroscopy is a valuable skill in organic chemistry. By understanding the characteristic IR absorptions of carboxylic acids and using the easy tips outlined in this article, you’ll be able to identify these compounds with confidence. Remember to always compare your IR spectra with known spectra and use the characteristic absorptions to confirm the identity of the compound.


