Interpreting Venous Blood Gases
Understanding venous blood gases (VBGs) is a crucial aspect of clinical practice, particularly in critical care and emergency medicine. VBGs provide valuable information about a patient’s oxygenation, ventilation, and acid-base balance. While arterial blood gases (ABGs) are often considered the gold standard, VBGs have become increasingly recognized for their utility in certain clinical contexts. This article delves into the interpretation of VBGs, exploring their components, clinical significance, and how they compare to ABGs.
Components of Venous Blood Gases
VBGs measure several key parameters:
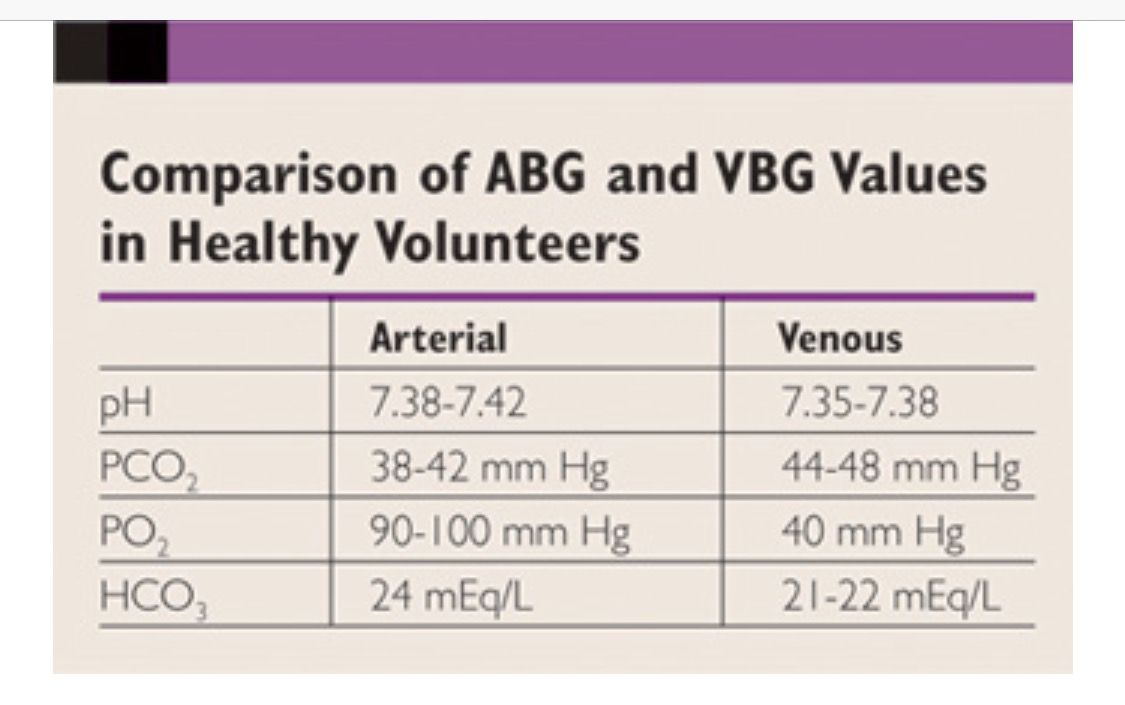
- pH: The measure of how acidic or alkaline the blood is, with a normal range of approximately 7.35 to 7.45.
- pCO2 (Partial Pressure of Carbon Dioxide): Reflects the level of carbon dioxide in the blood, with a normal range of about 40 to 50 mmHg in arterial blood but slightly higher in venous blood due to the ongoing metabolism of tissues.
- pO2 (Partial Pressure of Oxygen): Indicates the level of oxygen in the blood, though venous pO2 is not as commonly used for clinical decision-making as arterial pO2.
- Bicarbonate (HCO3-): A measure of the bicarbonate ion level in the blood, crucial for assessing the metabolic component of acid-base disturbances.
- Base Excess (BE): Provides information about the amount of base (or acid) that needs to be added to the blood to return the pH to normal, taking into account the hemoglobin level.
- Lactate: Often measured in conjunction with VBGs, especially in critically ill patients, to assess tissue perfusion and potential lactic acidosis.
Clinical Significance of Venous Blood Gases
VBGs are particularly useful in assessing:
- Tissue Perfusion: Through lactate levels and the base deficit, VBGs can provide clues about the adequacy of tissue perfusion and oxygenation.
- Metabolic Acidosis: An increased anion gap or the presence of lactic acidosis can be identified, guiding the management of conditions like sepsis or diabetic ketoacidosis.
- Respiratory Status: While pCO2 levels in venous blood are not directly equivalent to arterial levels, significant deviations can indicate problems with ventilation.
Comparison with Arterial Blood Gases
ABGs are drawn from an artery and provide a snapshot of the blood’s oxygenation and carbon dioxide levels as they enter the body’s tissues. The key differences between ABGs and VBGs lie in their sampling sites and the parameters they emphasize:
- Oxygenation: ABGs are better for assessing oxygenation status because they directly measure the oxygen levels being delivered to tissues.
- Ventilation: Both ABGs and VBGs can assess ventilation through pCO2 levels, but arterial levels are more reflective of alveolar ventilation.
- Metabolic Status: VBGs, especially when including lactate, are valuable for evaluating tissue perfusion and metabolic acid-base status.
Interpreting Venous Blood Gases
Interpreting VBGs involves understanding the context in which they are drawn and considering the following steps:
- Assess pH: Determine if the patient is acidotic (pH < 7.35) or alkalotic (pH > 7.45).
- Evaluate pCO2: In the context of VBGs, significantly elevated pCO2 levels may indicate respiratory acidosis, but this must be interpreted with caution due to the venous nature of the sample.
- Consider Bicarbonate and Base Excess: These parameters help in identifying and quantifying metabolic acid-base disturbances.
- Lactate Levels: Elevated lactate can indicate tissue hypoxia, severe sepsis, or other conditions leading to anaerobic metabolism.
Practical Applications and Limitations
VBGs are not a replacement for ABGs in all situations but offer a useful alternative in specific clinical scenarios, such as:
- Resource-Limited Settings: Where arterial sampling is not feasible.
- Serial Monitoring: For tracking changes in a patient’s condition over time, particularly in critically ill patients.
- Minimally Invasive: Less painful and potentially less risky than arterial puncture.
However, it’s crucial to understand the limitations, including the potential for slightly different reference ranges compared to ABGs and the need for careful interpretation in the context of the patient’s overall clinical picture.
Conclusion
Venous blood gases provide a valuable tool for clinicians, offering insights into oxygenation, ventilation, and metabolic status. While they should be interpreted with an understanding of their limitations and differences from arterial blood gases, VBGs can play a critical role in patient management, especially in scenarios where arterial sampling is challenging or unnecessary. As with any diagnostic tool, their utility is maximized when interpreted in the context of the patient’s complete clinical profile.
What is the primary advantage of using venous blood gases over arterial blood gases in clinical practice?
+The primary advantage of using venous blood gases (VBGs) is their less invasive nature and the potential for serial monitoring without the risks associated with arterial puncture. This makes VBGs particularly useful in resource-limited settings or for continuous assessment in critically ill patients.
How do venous and arterial blood gas results differ, and what are the implications for clinical interpretation?
+Venous blood gases (VBGs) and arterial blood gases (ABGs) differ primarily in their oxygen and carbon dioxide levels due to the extraction of oxygen and addition of carbon dioxide by tissues. ABGs are more reflective of alveolar ventilation and oxygenation status, while VBGs are useful for assessing tissue perfusion and metabolic acid-base status. The choice between VBGs and ABGs depends on the specific clinical question and the patient’s condition.
What role do lactate levels play in the interpretation of venous blood gases, and what do elevated levels indicate?
+Lactate levels are a critical component when interpreting venous blood gases, particularly in the context of critically ill patients. Elevated lactate levels can indicate tissue hypoperfusion, severe sepsis, or other conditions leading to anaerobic metabolism. The presence of hyperlactatemia should prompt further investigation into its cause and guide therapeutic interventions aimed at improving tissue oxygenation and perfusion.