Ionization Energy And Periodic Table
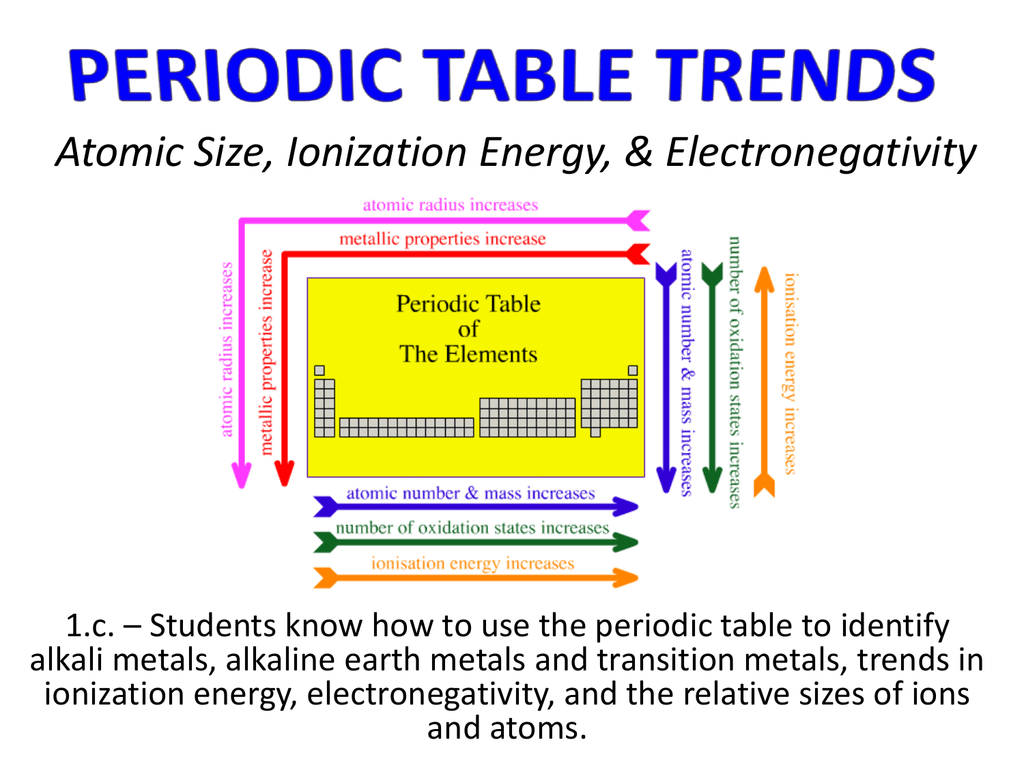
Ionization energy is a fundamental concept in chemistry that refers to the amount of energy required to remove an electron from a neutral atom in its ground state. This energy is typically measured in units of kJ/mol or eV, and it is an essential property of atoms that helps us understand the periodic trends and behavior of elements.
The periodic table is a tabular arrangement of the elements, organized by their atomic number, electron configuration, and recurring chemical properties. The elements are listed in order of increasing atomic number, which is the number of protons present in the nucleus of an atom. The periodic table is divided into rows called periods and columns called groups or families.
One of the most significant relationships between ionization energy and the periodic table is the periodic trend of ionization energy. As we move from left to right across a period, the ionization energy increases. This is because the number of protons in the nucleus increases, which leads to a greater attraction between the nucleus and the electrons. As a result, it becomes more difficult to remove an electron from the atom, and the ionization energy increases.
On the other hand, as we move down a group, the ionization energy decreases. This is because the number of energy levels or electron shells increases, which leads to a greater screening effect between the nucleus and the outermost electrons. The screening effect reduces the effective nuclear charge, making it easier to remove an electron from the atom.
The trend of ionization energy is not always straightforward and can be influenced by other factors such as electron configuration and the presence of half-filled or completely filled subshells. For example, the ionization energy of oxygen is lower than that of nitrogen, despite oxygen being to the right of nitrogen in the periodic table. This is because oxygen has a half-filled subshell, which leads to a higher stability and a lower ionization energy.
To illustrate the relationship between ionization energy and the periodic table, let’s consider the following examples:
| Element | Ionization Energy (kJ/mol) |
|---|---|
| Lithium (Li) | 520.2 |
| Sodium (Na) | 495.8 |
| Potassium (K) | 418.8 |
| Rubidium (Rb) | 403.0 |
| Caesium (Cs) | 375.7 |

As we can see from the table, the ionization energy decreases as we move down the group. This is because the number of energy levels increases, leading to a greater screening effect and a lower ionization energy.
In contrast, let’s consider the following examples of elements in the same period:
| Element | Ionization Energy (kJ/mol) |
|---|---|
| Boron (B) | 800.6 |
| Carbon © | 1086.5 |
| Nitrogen (N) | 1402.3 |
| Oxygen (O) | 1313.9 |
| Fluorine (F) | 1681.0 |
As we move from left to right across the period, the ionization energy increases. This is because the number of protons in the nucleus increases, leading to a greater attraction between the nucleus and the electrons.
The ionization energy of an element is influenced by its position in the periodic table. The ionization energy increases as we move from left to right across a period and decreases as we move down a group. Understanding these trends is essential for predicting the behavior of elements and their compounds.
Ionization energy is an important concept in chemistry that has numerous applications in various fields, including physics, materials science, and biology. By understanding the relationship between ionization energy and the periodic table, we can gain insights into the properties and behavior of elements and their compounds.
What is ionization energy, and why is it important?
+
How does ionization energy change as we move across a period?
+
What is the relationship between ionization energy and electron configuration?
+
In conclusion, the relationship between ionization energy and the periodic table is a complex and multifaceted one. By understanding the trends and patterns of ionization energy, we can gain insights into the properties and behavior of elements and their compounds. The periodic table provides a powerful framework for organizing and understanding the elements, and the study of ionization energy is an essential part of this framework.
Advantages and Disadvantages of Using Ionization Energy to Predict Chemical Reactivity

Advantages
- Ideal for predicting the reactivity of elements and their compounds
- Provides a quantitative measure of the energy required to remove an electron from an atom
- Helps to identify trends and patterns in the periodic table
Disadvantages
- Does not take into account other factors that influence chemical reactivity, such as electron affinity and lattice energy
- Can be difficult to measure accurately, especially for elements with high ionization energies
- May not provide a complete picture of the chemical behavior of an element or its compounds
The study of ionization energy and its relationship to the periodic table is a rich and complex field that continues to evolve and grow. By understanding the trends and patterns of ionization energy, we can gain insights into the properties and behavior of elements and their compounds, and develop new materials and technologies that exploit these properties.
In the future, researchers and scientists will continue to explore the relationship between ionization energy and the periodic table, using advanced computational methods and experimental techniques to probe the properties of atoms and molecules. As our understanding of ionization energy and its role in chemistry continues to grow, we can expect to see new breakthroughs and discoveries that will shape the course of science and technology in the years to come.
How to Calculate Ionization Energy

- Determine the atomic number of the element
- Identify the electron configuration of the element
- Calculate the number of protons in the nucleus
- Use the formula for ionization energy to calculate the energy required to remove an electron from the atom
- Compare the calculated ionization energy with the experimentally measured value to ensure accuracy
By following these steps and using the principles of ionization energy and the periodic table, researchers and scientists can gain a deeper understanding of the properties and behavior of elements and their compounds, and develop new materials and technologies that exploit these properties.

