Is Nacl A Covalent Compound
Sodium chloride (NaCl), commonly known as table salt, is a quintessential example of an ionic compound, not a covalent compound. This distinction is fundamental in chemistry and hinges on the nature of the chemical bonds holding the compound together. Let’s delve into why NaCl is ionic, explore the differences between ionic and covalent compounds, and address common misconceptions.
The Nature of Chemical Bonds in NaCl
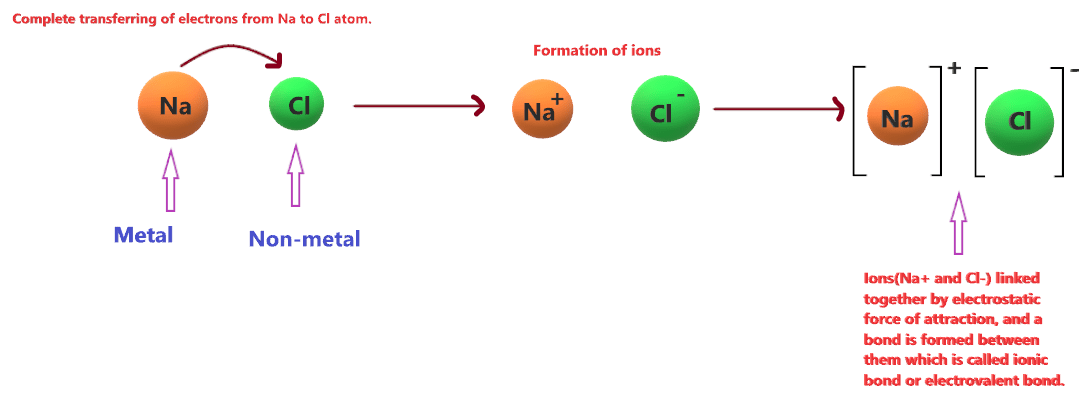
NaCl is composed of sodium (Na), a highly reactive metal from Group 1 of the periodic table, and chlorine (Cl), a halogen from Group 17. The bond between these two elements is ionic, which arises due to the transfer of electrons from sodium to chlorine. Here’s how it works:
- Electron Transfer: Sodium has one valence electron (in its 3s orbital), which it readily donates to achieve a stable electron configuration like that of neon. Chlorine, on the other hand, needs one more electron to complete its octet and achieve the stable configuration of argon.
- Formation of Ions: When sodium donates its electron to chlorine, it becomes a positively charged sodium ion (Na⁺), and chlorine becomes a negatively charged chloride ion (Cl⁻).
- Electromagnetic Attraction: The oppositely charged ions are held together by strong electrostatic forces, forming a crystalline lattice structure characteristic of ionic compounds.
Key Differences Between Ionic and Covalent Compounds
To understand why NaCl is not covalent, it’s essential to contrast ionic and covalent bonds:
Why NaCl is Not a Covalent Compound
Covalent compounds involve the sharing of electrons between atoms, typically between nonmetals. In NaCl, there is no electron sharing; instead, there is a complete transfer of electrons from sodium to chlorine. This transfer results in the formation of ions, which is the hallmark of an ionic bond.
Additionally, the electronegativity difference between sodium (0.93) and chlorine (3.16) is 2.23, well above the threshold of 1.7 that typically defines ionic bonds. This large difference ensures that the bond is ionic rather than covalent.
Practical Implications of NaCl’s Ionic Nature
- High Melting Point: The strong electrostatic forces between Na⁺ and Cl⁻ ions require significant energy to break, resulting in a high melting point (801°C or 1474°F).
- Solubility in Water: NaCl dissolves readily in water because water molecules, being polar, can surround and separate the ions (a process called solvation).
- Electrical Conductivity: In its solid state, NaCl does not conduct electricity because the ions are fixed in the lattice. However, when melted or dissolved in water, the free ions allow for electrical conduction.
Common Misconceptions
“NaCl has a polar bond, so it’s covalent.”
Polarity refers to the separation of charge within a bond. While ionic bonds are inherently polar due to the complete transfer of electrons, covalent bonds can also be polar if electrons are shared unequally. However, the key difference lies in the type of bonding (transfer vs. sharing).“All compounds with metals are ionic.”
While many metal-nonmetal compounds are ionic, some, like aluminum chloride (AlCl₃), exhibit covalent characteristics due to the smaller size and higher charge of the metal ion, which can polarize the electron cloud of the nonmetal.
Expert Insight
Understanding the ionic nature of NaCl is crucial not only in chemistry but also in fields like biology and materials science. For instance, the dissociation of NaCl in biological fluids is essential for nerve impulse transmission and cellular function. Its crystalline structure also serves as a model for understanding other ionic materials, such as those used in batteries and semiconductors.
FAQ Section
Can NaCl form covalent bonds under any conditions?
+No, NaCl inherently forms ionic bonds due to the large electronegativity difference between sodium and chlorine. Even under extreme conditions, the bond remains ionic.
Why does NaCl dissolve in water but not in nonpolar solvents?
+NaCl dissolves in water because water molecules can surround and separate the ions (solvation). Nonpolar solvents lack the polarity to interact effectively with the ions, so NaCl remains undissolved.
How does the ionic nature of NaCl affect its role in cooking?
+The ionic nature of NaCl allows it to dissolve easily in food moisture, enhancing flavor by releasing Na⁺ and Cl⁻ ions. Its high melting point also ensures stability during cooking.
Are there any covalent compounds with similar properties to NaCl?
+No, covalent compounds lack the ionic lattice structure and properties like high melting points and electrical conductivity in aqueous solutions. However, some polar covalent compounds, like sugar, dissolve in water due to their polarity.
Conclusion
In summary, NaCl is unequivocally an ionic compound, not a covalent one. Its properties—high melting point, solubility in water, and electrical conductivity in molten or aqueous states—stem from the electrostatic forces between Na⁺ and Cl⁻ ions. Understanding this distinction is fundamental in chemistry and has wide-ranging applications in science and everyday life. Whether in the lab, kitchen, or industrial processes, the ionic nature of NaCl plays a pivotal role in its behavior and utility.