Molar Mass For Koh
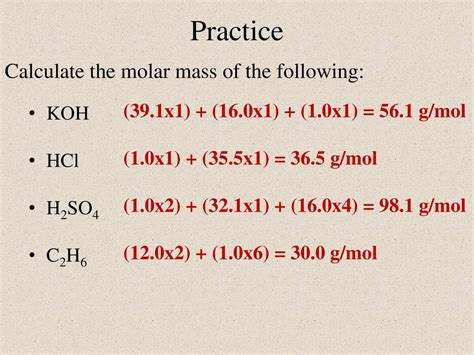
The concept of molar mass is fundamental in chemistry, as it allows us to understand the relationship between the mass of a substance and the number of particles it contains. When discussing the molar mass of KOH, we’re referring to potassium hydroxide, a strong base commonly used in various industrial and laboratory applications. To calculate the molar mass of KOH, we need to sum the atomic masses of its constituent elements: potassium (K), oxygen (O), and hydrogen (H).
The atomic mass of potassium (K) is approximately 39.09 g/mol. Oxygen (O) has an atomic mass of about 16.00 g/mol, and hydrogen (H) has an atomic mass of approximately 1.01 g/mol. By adding these masses together, we can calculate the molar mass of KOH:
Molar mass of KOH = Atomic mass of K + Atomic mass of O + Atomic mass of H Molar mass of KOH = 39.09 g/mol + 16.00 g/mol + 1.01 g/mol Molar mass of KOH = 56.10 g/mol
Thus, the molar mass of KOH is approximately 56.10 grams per mole. This value is crucial for calculating the number of moles of KOH in a given sample, which in turn is essential for preparing solutions of known concentration, a common task in chemical experiments and industrial processes.
Understanding Molar Mass and Its Importance
The molar mass of a compound is a measure of the mass of one mole of that compound. One mole of any substance contains 6.022 x 10^23 particles (atoms or molecules), known as Avogadro’s number. The molar mass is a bridge between the microscopic world of atoms and molecules and the macroscopic world of grams and liters that we can measure directly in the laboratory.
Applications of KOH
Potassium hydroxide, with its high molar mass accuracy, is used in a variety of applications:
- Manufacture of Soap and Detergents: KOH is used in the saponification process to produce soft soaps.
- Battery Production: It serves as the electrolyte in alkaline batteries.
- Food Industry: KOH is used in the production of foods like chocolate and soft drinks.
- Laboratory Settings: It is a common reagent in various chemical experiments, including titrations to determine the concentration of acids.
Practical Calculation of KOH
In practical scenarios, knowing the molar mass of KOH is crucial for making precise chemical calculations. For instance, if you need to prepare a solution of KOH with a specific concentration, you would use its molar mass to calculate how much KOH to dissolve in a solvent to achieve the desired molarity (moles of solute per liter of solution).
Calculating Molarity of KOH Solution
To prepare a 1 M (1 mole per liter) solution of KOH, you would need to weigh out the amount of KOH that corresponds to one mole, which is 56.10 grams, and dissolve it in enough water to make one liter of solution.
Steps to Prepare KOH Solution
- Determine the Molarity Needed: Decide on the concentration (molarity) of the KOH solution you want to prepare.
- Calculate the Mass of KOH Needed: Use the formula: Mass of KOH = Molarity * Molar mass of KOH * Volume of solution (in liters).
- Weigh Out KOH: Accurately weigh out the calculated amount of KOH using a balance.
- Dissolve in Solvent: Slowly add the weighed KOH to a solvent (like water), stirring until it’s completely dissolved.
- Adjust to Final Volume: Add more solvent as necessary to reach the desired final volume.
Historical Evolution of KOH Usage
The use of potassium hydroxide dates back centuries, with early applications in the production of soap from animal fat and olive oil. Over time, as chemistry evolved and the properties of KOH became better understood, its applications expanded to include electrochemistry, where it plays a critical role as an electrolyte, enhancing the efficiency of battery cells.
Future Trends and Developments
As the demand for sustainable and efficient technologies increases, the role of KOH in emerging fields such as renewable energy storage and advanced materials synthesis is expected to grow. Research into more efficient and safer methods of producing and handling KOH, as well as exploring its potential in novel applications, will continue to drive innovation.
Comparative Analysis with Other Alkalis
When comparing KOH with other strong bases like sodium hydroxide (NaOH), it’s essential to consider not just their chemical properties but also their industrial applications and economic factors. While NaOH is more commonly used in certain processes due to its availability and lower cost, KOH offers specific advantages in applications where its unique properties, such as lower melting point and different solubility characteristics, are beneficial.
Balanced View: Advantages and Disadvantages of KOH
Advantages: - Highly soluble in water, making it useful for aqueous solutions. - Lower melting point compared to NaOH, which can be advantageous in certain applications. - Can be used in the production of biodiesel, showcasing its role in renewable energy.
Disadvantages: - Generally more expensive than NaOH. - Requires careful handling due to its caustic nature, posing risks to skin and eyes. - Environmental considerations must be taken into account, as improper disposal can harm aquatic life.
Myth vs. Reality: Safety Concerns with KOH
There’s a prevailing myth that strong bases like KOH are less dangerous than strong acids because they feel slippery. The reality is that KOH is highly caustic and can cause severe chemical burns upon contact with skin or eyes, necessitating proper protective gear when handling it.
Conclusion
In conclusion, the molar mass of KOH, approximately 56.10 g/mol, is a fundamental piece of information for both theoretical understanding and practical applications of this chemical compound. Whether in industrial manufacturing, laboratory research, or educational contexts, grasping the concept of molar mass and its implications for the preparation and use of KOH solutions is crucial. As we move forward, the versatility and essential nature of KOH in advancing technologies will continue to highlight its importance in the chemical sciences.
FAQ Section
What is the molar mass of KOH and how is it calculated?
+The molar mass of KOH is approximately 56.10 g/mol, calculated by summing the atomic masses of potassium (39.09 g/mol), oxygen (16.00 g/mol), and hydrogen (1.01 g/mol).
What are some common applications of KOH?
+KOH is used in the manufacture of soap and detergents, as an electrolyte in alkaline batteries, and in various food and laboratory applications.
How do I prepare a 1 M solution of KOH?
+To prepare a 1 M solution of KOH, weigh out 56.10 grams of KOH and dissolve it in enough water to make one liter of solution.
What safety precautions should be taken when handling KOH?
+KOH is highly caustic and requires careful handling. Wear protective gear, including gloves and goggles, and avoid skin and eye contact to prevent chemical burns.
