Molecular Geometry Of Hbr
The molecular geometry of HBr, or hydrogen bromide, is a fundamental concept in chemistry that helps us understand the arrangement of atoms in a molecule and its resulting physical and chemical properties. To delve into this topic, we first need to understand the basics of molecular geometry and the factors that influence it.
Molecular geometry is determined by the interaction of electron pairs around the central atom of a molecule. These interactions can be between bonding pairs (electrons involved in covalent bonds between atoms) and lone pairs (electrons not involved in bonding). The theory that best explains how these electron pairs arrange themselves around a central atom is the Valence Shell Electron Pair Repulsion (VSEPR) theory. According to VSEPR, electron pairs repel each other due to their negative charge, and they will arrange themselves to maximize the distance between them, thus minimizing repulsion.
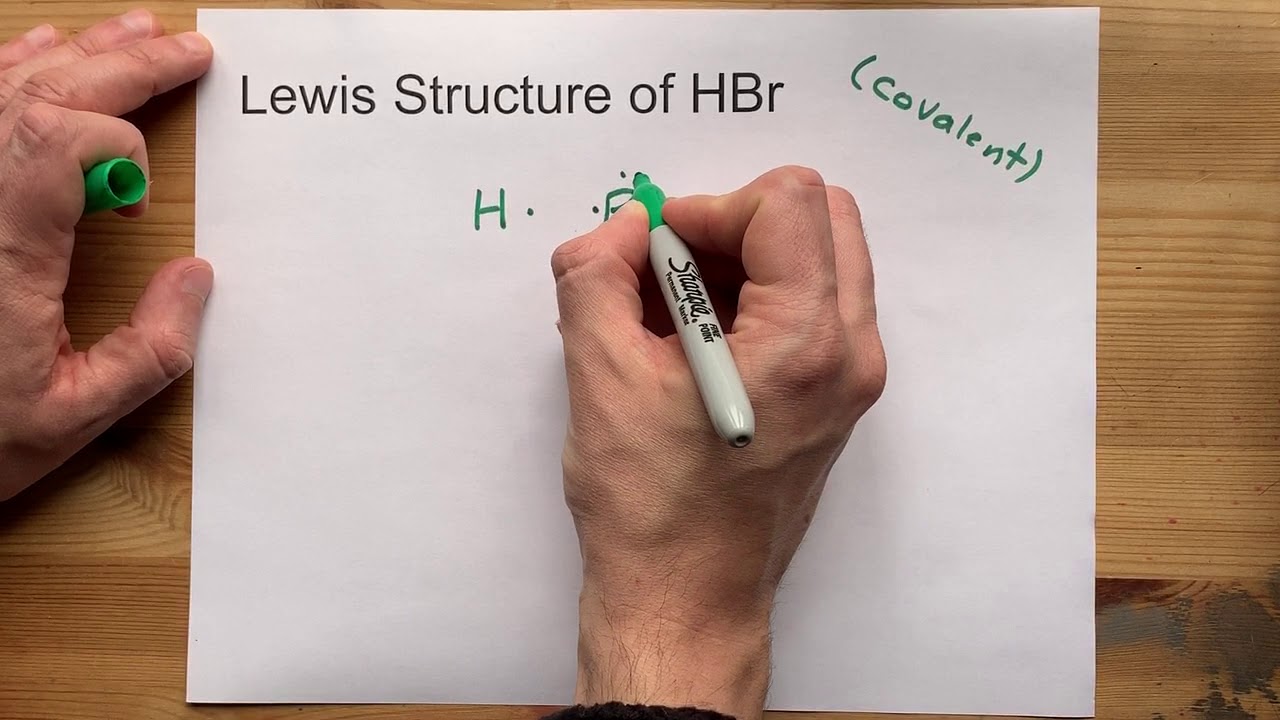
In the case of HBr, we have a molecule composed of one hydrogen atom (H) and one bromine atom (Br). Hydrogen has one valence electron, and bromine has seven valence electrons. When these two atoms form a covalent bond, they share a pair of electrons, resulting in a single bond between them. The hydrogen atom achieves a stable configuration with two electrons (a duet), while the bromine atom, with six remaining valence electrons after the bond formation, has three lone pairs.
Given this arrangement, the central atom in our consideration is bromine (Br), as it is the atom to which the hydrogen is bonded and around which we are examining the geometry. Since there is one bonding pair (the H-Br bond) and three lone pairs on the bromine atom, we apply the VSEPR theory to predict the geometry.
The VSEPR theory states that four electron pairs (whether bonding or lone pairs) around a central atom will arrange themselves in a tetrahedral geometry to maximize the distance between them. However, when considering the actual shape or molecular geometry of the molecule, we look only at the positions of the atoms, not the lone pairs. In the case of HBr, we have one bonding pair and three lone pairs around the bromine atom.
The arrangement of these electron pairs is tetrahedral because it represents the most stable geometry that minimizes electron pair repulsions. However, since we only have one atom (hydrogen) bonded to the bromine and three lone pairs, the molecular geometry—the shape considering only the atoms—of HBr is linear or, more specifically, it is considered to be “bent” or V-shaped if we were to visualize the lone pairs. But, in the context of describing the molecular shape based solely on the positions of atoms, HBr is often simply described as having a linear geometry due to the direct bond between H and Br, despite the bent nature considering electron pairs.
It’s essential to note, however, that in the strictest sense regarding molecular geometry based on atomic positions, HBr is linear because it has only two atoms, and any two points are always collinear (on the same line). The bent description would more accurately apply if we were discussing the electron pair geometry around the bromine atom, including both bonding and lone pairs.
The molecular geometry of HBr, like many diatomic molecules (molecules composed of two atoms), is thus often simplified as linear. This simplicity stems from the molecule’s composition of only two atoms, which inherently suggests a straight-line arrangement. The presence of lone pairs on the bromine atom, while influencing the electron pair geometry, does not change the fact that, from a purely atomic positional standpoint, HBr is linear.
Understanding the molecular geometry of molecules like HBr is crucial for predicting their physical properties, such as polarity, melting and boiling points, and their chemical reactivity. For HBr, its polarity arises from the difference in electronegativity between hydrogen and bromine, with bromine being more electronegative. This polarity can significantly influence the molecule’s behavior in various chemical environments and its interactions with other molecules.
In conclusion, the molecular geometry of HBr, based on the arrangement of atoms, is linear. The VSEPR theory provides a framework for understanding how electron pairs arrange themselves around a central atom, but in the case of diatomic molecules like HBr, the simplicity of having only two atoms results in a linear molecular geometry. This understanding is foundational in chemistry and is crucial for explaining a wide range of physical and chemical properties of molecules.
What is the molecular geometry of HBr based on the positions of atoms?
+The molecular geometry of HBr, based on the positions of atoms, is linear.
How does the VSEPR theory apply to the electron pairs around the bromine atom in HBr?
+The VSEPR theory states that the four electron pairs (one bonding pair and three lone pairs) around the bromine atom in HBr will arrange themselves in a tetrahedral geometry to maximize the distance between them and minimize repulsion.
What is the significance of understanding the molecular geometry of HBr?
+Understanding the molecular geometry of HBr is crucial for predicting its physical properties, such as polarity, and its chemical reactivity, which are influenced by the arrangement of electrons and atoms within the molecule.