Molecules Into Moles
The concept of converting molecules into moles is a fundamental principle in chemistry, serving as a bridge between the microscopic world of molecules and the macroscopic world of laboratory measurements. This conversion is crucial for calculating the amounts of substances involved in chemical reactions, which is essential for predicting outcomes, designing experiments, and scaling up reactions from the laboratory to industrial levels.
Understanding Molecules and Moles
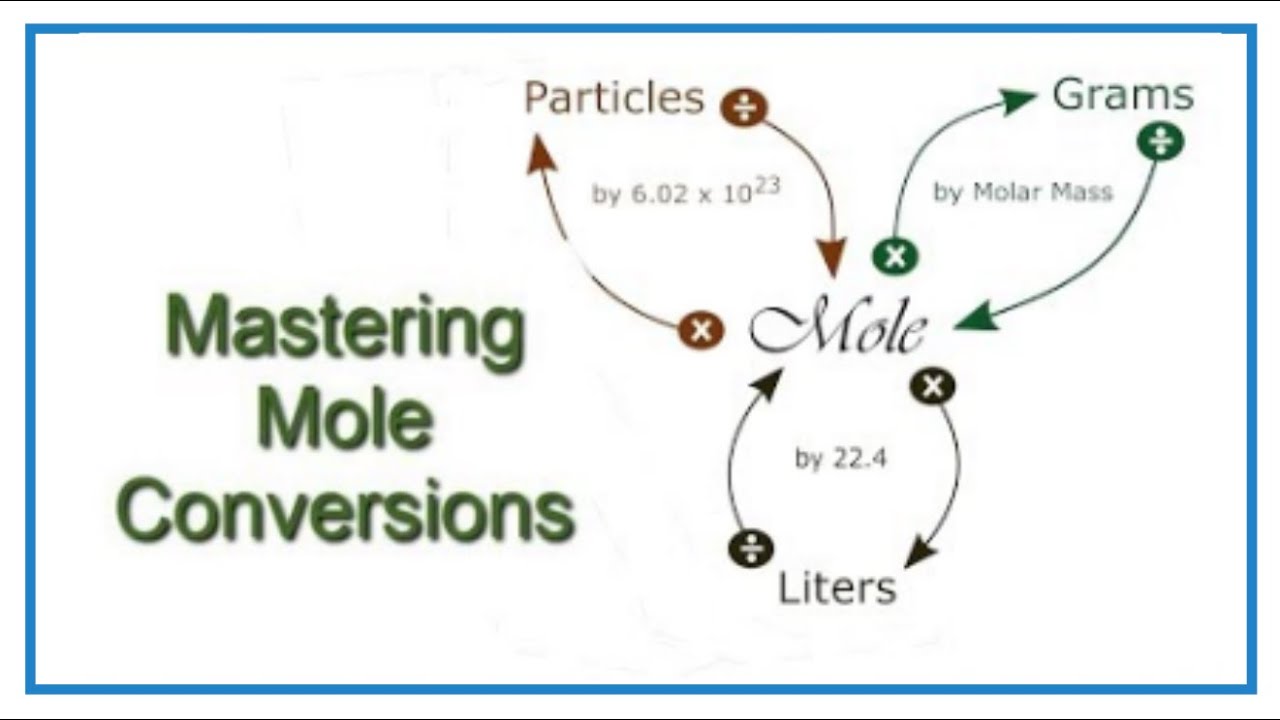
At the heart of chemistry is the molecule, which is the smallest unit of a substance that retains the properties of that substance. Molecules are composed of atoms, and the number of molecules in a sample can be staggering, often exceeding billions or even trillions. To manage these large numbers, chemists use the concept of the mole, which is defined as 6.022 x 10^23 particles (molecules, atoms, ions, etc.). This number is known as Avogadro’s number.
The Role of Molecular Weight
Converting molecules into moles involves understanding the molecular weight of a substance. The molecular weight (or molecular mass) is the sum of the atomic weights of the atoms in a molecule. It serves as a conversion factor between the mass of a substance and the number of moles. For instance, if you know the mass of a sample and its molecular weight, you can calculate the number of moles of that substance in the sample.
Calculation Steps
To convert molecules into moles, follow these steps:
Determine the Molecular Weight: First, calculate the molecular weight of the substance. For example, water (H2O) has a molecular weight of approximately 18 grams/mole because hydrogen (H) has an atomic weight of about 1 gram/mole and oxygen (O) has an atomic weight of about 16 grams/mole.
Know the Mass: Have the mass of the substance you are working with. This could be given in grams, kilograms, or any other unit of mass.
Apply the Formula: Use the formula
moles = mass / molecular weightto calculate the number of moles. For example, if you have 36 grams of water, you would calculate the number of moles as follows:moles = 36 grams / 18 grams/mole = 2 moles.
Practical Applications
The ability to convert between molecules and moles has numerous practical applications:
- Chemical Reactions: In chemical reactions, reactants and products are often measured in moles to ensure stoichiometric ratios are maintained, which is crucial for efficiency, yield, and safety.
- Pharmaceuticals: The active ingredients in drugs are measured in moles to ensure precise dosages.
- Environmental Science: The concentration of pollutants or nutrients in water and soil can be expressed in moles per liter or per kilogram, helping in the assessment and management of environmental quality.
Challenges and Considerations
While the concept of converting molecules to moles is straightforward, several challenges and considerations arise in practical applications:
- Purity of Substances: The calculation assumes pure substances. Impurities can affect the accuracy of molecular weight and, consequently, the mole calculation.
- Measurement Errors: Small errors in measuring the mass of a substance can lead to significant errors in calculating the number of moles, especially when dealing with small quantities.
- Complexity of Mixtures: In mixtures, knowing the composition is crucial for calculating the number of moles of each component.
Future Directions
As technology advances, the precision with which we can measure and manipulate molecules continues to improve. Techniques such as mass spectrometry allow for the precise measurement of molecular weights, and advances in microchemistry enable the handling of extremely small quantities of substances, pushing the boundaries of what is possible in terms of mole-to-molecule conversions.
Conclusion
The conversion of molecules into moles is a foundational concept in chemistry, bridging the microscopic and macroscopic worlds. Understanding this conversion is vital for a wide range of applications, from chemical synthesis and environmental monitoring to pharmaceutical development. As science continues to evolve, the importance of precise mole-to-molecule conversions will only continue to grow, underpinning advancements in fields that rely on the accurate manipulation of matter at its most fundamental level.
What is the significance of Avogadro’s number in converting molecules to moles?
+Avogadro’s number (6.022 x 10^23 particles) serves as a conversion factor between the amount of a substance measured in grams and the amount measured in moles. It allows chemists to relate the mass of a substance to the number of molecules, facilitating calculations and predictions in chemical reactions.
How does molecular weight affect the conversion of molecules to moles?
+The molecular weight of a substance is crucial for converting between molecules and moles. It is used as a conversion factor where the mass of the substance divided by its molecular weight yields the number of moles. Different substances have different molecular weights due to the varying atomic weights of their constituent atoms, affecting how many moles are in a given mass of the substance.
What are some common challenges in converting molecules to moles in practical applications?
+Common challenges include ensuring the purity of the substances (as impurities can affect molecular weight calculations), minimizing measurement errors (as small errors can lead to significant discrepancies in mole calculations), and accurately determining the composition of mixtures (to correctly calculate the number of moles of each component).

