Reactive Nonmetals

In the vast tapestry of the periodic table, reactive nonmetals stand out as a fascinating group of elements that bridge the gap between metals and nonmetals. These elements, often referred to as halogens and noble gases, exhibit unique chemical behaviors, physical properties, and applications that make them indispensable in various fields. This article delves into the world of reactive nonmetals, exploring their characteristics, reactivity, applications, and their pivotal role in shaping our understanding of chemistry.
Understanding Reactive Nonmetals: A Chemical Overview
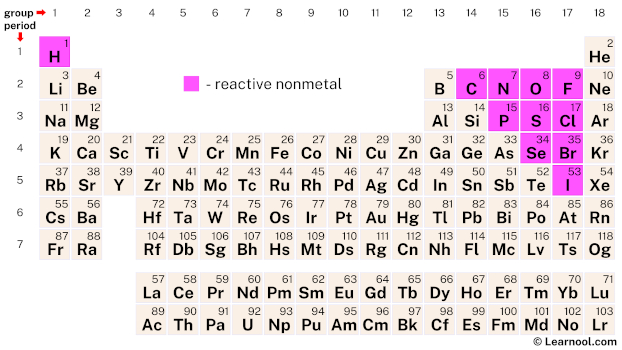
Reactive nonmetals are primarily found in Group 17 (halogens) and Group 18 (noble gases) of the periodic table. Halogens, including fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At), are highly reactive elements known for their electronegativity and tendency to form salts. Noble gases, such as helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), are characterized by their full valence electron shells, making them generally unreactive under normal conditions.
Expert Insight: The reactivity of halogens decreases down the group due to the increasing atomic radius, which reduces the effective nuclear charge and makes it harder to attract electrons.
The Halogens: Masters of Reactivity
Halogens are among the most reactive nonmetals, owing to their high electronegativity and strong desire to gain one electron to achieve a stable electron configuration. This reactivity is evident in their ability to form halide ions (e.g., F⁻, Cl⁻) and compounds such as sodium chloride (NaCl) and hydrogen chloride (HCl).
Reactivity of Halogens: Pros and Cons
- Pros: High reactivity allows halogens to be used in disinfection (e.g., chlorine in water treatment), pharmaceuticals, and industrial processes.
- Cons: Their reactivity can be hazardous, requiring careful handling to avoid accidents or environmental damage.
Noble Gases: The Inert Paradox
Noble gases, despite being classified as nonmetals, are often considered inert due to their full valence electron shells. However, under specific conditions, some noble gases, particularly xenon and krypton, can form compounds with highly electronegative elements like fluorine and oxygen.
"The discovery of noble gas compounds challenged the long-held belief that these elements were completely inert, opening new avenues in chemical research."
Applications of Reactive Nonmetals
The unique properties of reactive nonmetals lend themselves to a wide range of applications across industries:
Key Applications:
- Halogens in Disinfection: Chlorine is widely used in water treatment to kill bacteria and viruses, ensuring safe drinking water.
- Noble Gases in Lighting: Neon and argon are used in fluorescent lamps and lasers due to their ability to emit light when electrified.
- Fluorine in Pharmaceuticals: Fluorine-containing compounds are essential in the development of drugs, including antibiotics and anti-cancer agents.
- Xenon in Medical Imaging: Xenon is used as a contrast agent in MRI scans and as an anesthetic in medical procedures.
Environmental and Safety Considerations
While reactive nonmetals are invaluable in various applications, their use comes with environmental and safety challenges. Halogens, for instance, can contribute to ozone depletion (e.g., chlorofluorocarbons) and pose health risks if mishandled. Noble gases, though generally inert, can displace oxygen in confined spaces, leading to asphyxiation.
Key Takeaway: Responsible use and regulation of reactive nonmetals are essential to mitigate their potential risks while harnessing their benefits.
Future Trends: Expanding the Horizons of Reactive Nonmetals
Advancements in chemistry continue to uncover new possibilities for reactive nonmetals. Research into halogen-based materials for energy storage, noble gas compounds for catalysis, and innovative applications in nanotechnology are paving the way for future breakthroughs.
Future Implications: As technology evolves, reactive nonmetals are poised to play a critical role in addressing global challenges, from sustainable energy to advanced medical treatments.
What makes halogens highly reactive?
+Halogens are highly reactive due to their high electronegativity and the need to gain one electron to achieve a stable electron configuration, resembling the nearest noble gas.
Can noble gases form compounds?
+Yes, under specific conditions, noble gases like xenon and krypton can form compounds with highly electronegative elements such as fluorine and oxygen.
What are the environmental risks of halogens?
+Halogens can contribute to ozone depletion and pose health risks if not handled properly, necessitating strict regulations and safety measures.
How are noble gases used in everyday life?
+Noble gases are used in lighting (e.g., neon signs, fluorescent lamps), medical imaging, and as shielding gases in welding and other industrial processes.
What is the role of fluorine in pharmaceuticals?
+Fluorine is crucial in pharmaceutical development, enhancing drug stability, bioavailability, and efficacy in treating various diseases.
Reactive nonmetals, with their distinct properties and versatile applications, remain a cornerstone of modern chemistry. From the reactivity of halogens to the inertness of noble gases, these elements continue to inspire scientific exploration and innovation. As we unlock their potential, it is imperative to balance their benefits with environmental and safety considerations, ensuring a sustainable future for generations to come.

