Silver And Chloride
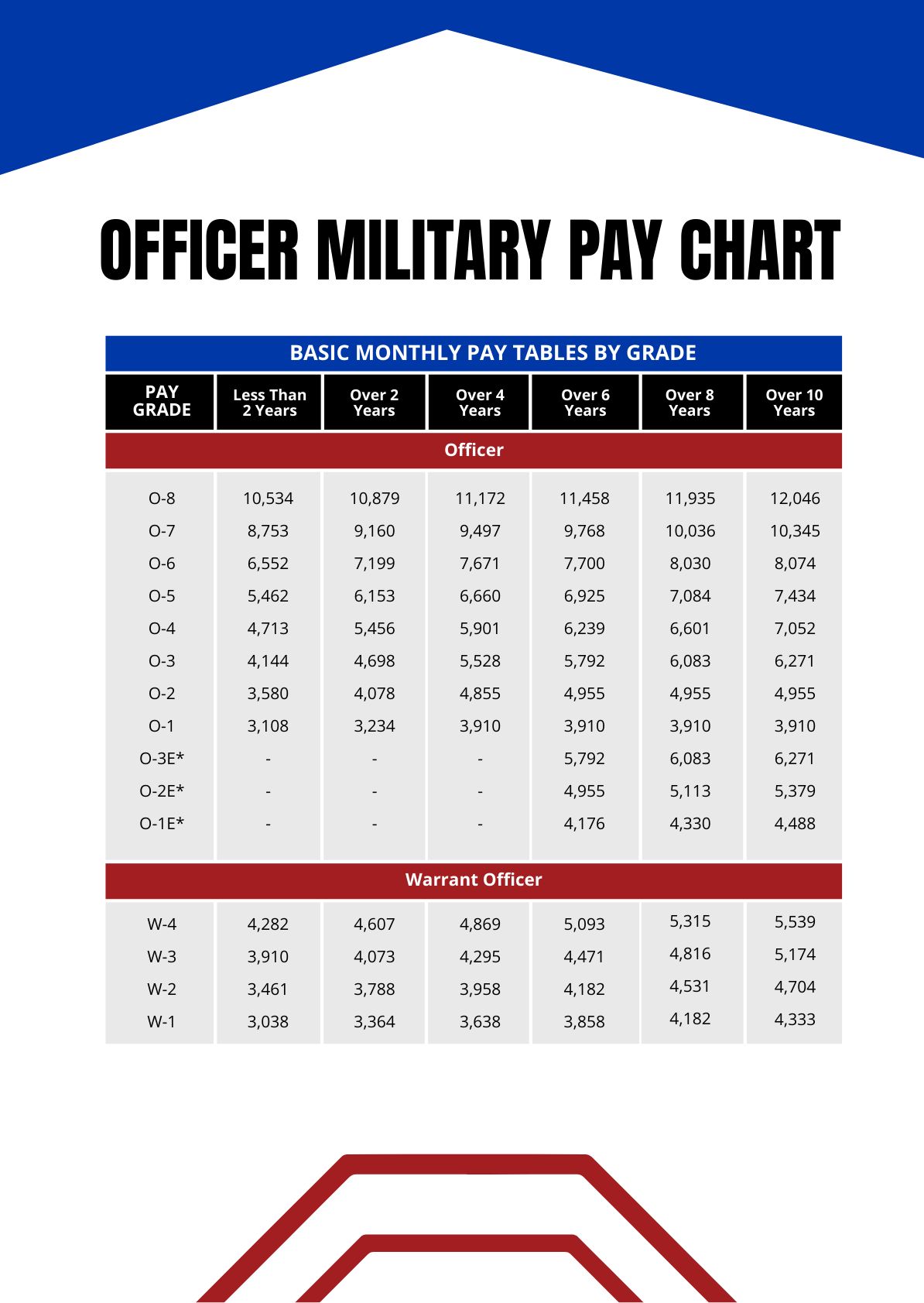
The combination of silver and chloride has been a subject of fascination for centuries, with applications ranging from photography to medicine. At the heart of this combination lies a unique chemical bond that has captivated scientists and craftsmen alike. To delve into the intriguing world of silver and chloride, it’s essential to understand the properties of these elements individually before exploring their compounds and applications.
Silver: A Precious Metal with Unique Properties
Silver, known by its chemical symbol Ag, is a soft, white, shiny metal. It is a member of the copper group of metals and is found in nature, usually in ores containing copper, lead, or gold. One of the most notable properties of silver is its high electrical conductivity; it is the best conductor of electricity among all elements, even surpassing copper. This property, combined with its malleability and ductility, makes silver an excellent material for various industrial applications, including electronics and solar panels.
Beyond its industrial uses, silver has also been valued for its antimicrobial properties. Silver ions (Ag+) have been shown to be toxic to a wide range of microorganisms, making silver an effective agent in wound dressings and water purification systems. This unique ability to combat bacteria, viruses, and fungi has led to the incorporation of silver in medical equipment, clothing, and even in some household items to reduce the spread of infections.
Chloride: A Ubiquitous Anion with Diverse Roles
Chloride, with the chemical symbol Cl-, is a halide anion. It is one of the most abundant ions in nature, found in various compounds, from table salt (sodium chloride, NaCl) to the chlorides of calcium, magnesium, and potassium, which are essential for many biological processes. Chloride ions play a critical role in maintaining fluid balance in the body and are crucial for the proper functioning of the nervous system. In the environment, chloride is a major component of seawater, influencing ocean chemistry and marine life.
In industrial and commercial settings, chlorides are used in a variety of applications, including as a component in the production of paper, dyes, and textiles. Chloride salts are also used in metal processing and as a reagent in various chemical reactions.
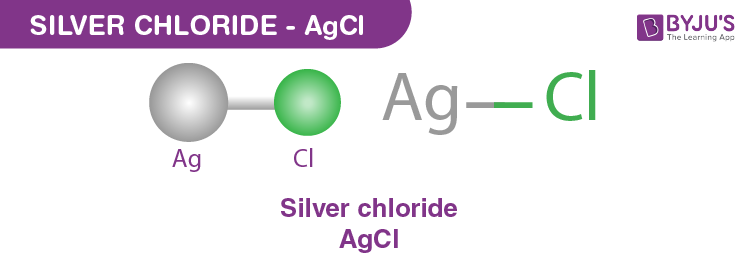
Silver Chloride: Formation and Properties
When silver reacts with chlorine or hydrochloric acid, it forms silver chloride (AgCl), a compound that is highly insoluble in water. This characteristic makes silver chloride useful in certain photographic processes and as an antibacterial agent. The formation of silver chloride is an example of a double displacement reaction, where silver reacts with chloride ions to form a precipitate:
Ag (silver) + Cl2 (chlorine) → AgCl2 (silver chloride), or in the presence of hydrochloric acid, Ag + HCl → AgCl + H.
Silver chloride’s low solubility in water is significant because it allows the compound to be used in applications where a controlled release of silver ions is desired, such as in antimicrobial coatings and wound care products.
Applications of Silver and Chloride
The unique properties of both silver and chloride have led to numerous applications across various fields:
- Photography: Historically, silver chloride was a crucial component in photographic films and papers, where it served as a light-sensitive material. When exposed to light, silver chloride decomposes into silver and chlorine, a process that was utilized to capture images.
- Medical Applications: The antimicrobial properties of silver make it an essential component in wound care, implantable medical devices, and even in some pharmaceuticals. Chloride ions, being essential for various bodily functions, are also a component of intravenous solutions and other medical treatments.
- Water Purification: Silver, often in the form of silver chloride, is used in water treatment systems to remove bacteria, viruses, and other pathogens, providing safe drinking water.
- Electronics: The high conductivity of silver makes it a preferred material for contacts and connectors in electronic devices. Chloride ions can also play a role in the manufacture of semiconductors and in electroplating processes.
Future Trends and Developments
As research continues to uncover new properties and applications of silver and chloride compounds, there’s a growing interest in exploring their potential in emerging technologies:
- Nanotechnology: Silver nanoparticles, often stabilized with chloride ions, are being studied for their enhanced antimicrobial properties and potential uses in biomedical applications, such as targeted drug delivery and sensing.
- Renewable Energy: The unique electrical properties of silver make it a critical component in solar panels. Additionally, chloride ions are being researched for their role in advanced battery technologies, such as chloride-ion batteries, which could offer improved performance and sustainability.
Conclusion
The combination of silver and chloride offers a wide range of applications, from traditional uses in photography and medicine to cutting-edge technologies in electronics and renewable energy. Understanding the chemical and physical properties of these elements and their compounds is key to harnessing their potential and developing innovative solutions for future challenges.
In the realm of science and technology, the exploration of silver and chloride continues to evolve, driven by the need for sustainable, effective, and safe materials and processes. As researchers delve deeper into the properties and applications of these elements, we can expect to see new and exciting developments that leverage the unique characteristics of silver and chloride to address global needs.
What are the primary uses of silver chloride?
+Silver chloride is used in photography, as an antibacterial agent, and in various chemical reactions due to its properties and the controlled release of silver ions.
Why is silver considered a good conductor of electricity?
+Silver is the best conductor of electricity among all elements due to its atomic structure, which allows for easy movement of electrons, thus facilitating the flow of electric current.
How do chloride ions contribute to human health?
+Chloride ions play a crucial role in maintaining fluid balance and are essential for the proper functioning of the nervous system. They also help in the digestion of food and are a component of stomach fluids.