Srbr2 Lewis Structure Made Easy
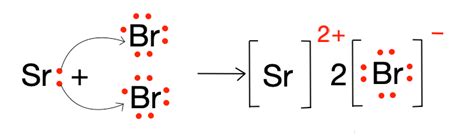
The SrBr2 Lewis structure is a fundamental concept in chemistry that represents the molecular geometry and bonding of strontium bromide. To understand this structure, we need to delve into the world of chemical bonding and electron configuration.
Introduction to Lewis Structures
Lewis structures, also known as electron dot diagrams, are a way to represent the valence electrons of atoms in a molecule. They provide a visual representation of the bonding between atoms and help predict the shape of a molecule. The Lewis structure is composed of dots, which represent the valence electrons, and lines, which represent the bonds between atoms.
Understanding SrBr2
Strontium bromide (SrBr2) is an ionic compound composed of strontium (Sr) and bromine (Br) atoms. Strontium is a Group 2 element, also known as an alkaline earth metal, while bromine is a Group 17 element, also known as a halogen. The combination of these two elements results in the formation of an ionic bond, where the strontium atom loses two electrons to form a positive ion (Sr2+), and the bromine atoms gain one electron each to form negative ions (Br-).
Drawing the SrBr2 Lewis Structure
To draw the SrBr2 Lewis structure, we need to follow a series of steps:
- Determine the central atom: In this case, the central atom is strontium (Sr).
- Calculate the total number of valence electrons: Strontium has two valence electrons, and each bromine atom has seven valence electrons. Since there are two bromine atoms, the total number of valence electrons is 2 (Sr) + 2 x 7 (Br) = 16.
- Draw the electrons around the central atom: Since strontium is the central atom, we draw its two valence electrons as dots around the atom.
- Add the bromine atoms: We add the two bromine atoms to the structure, each with seven valence electrons.
- Form the bonds: The strontium atom shares its two valence electrons with the two bromine atoms, forming two ionic bonds.
The resulting SrBr2 Lewis structure shows the strontium atom in the center, surrounded by two bromine atoms, with ionic bonds between them.
Key Takeaways
- The SrBr2 Lewis structure represents the ionic bonding between strontium and bromine atoms.
- The structure shows two ionic bonds between the strontium atom and the two bromine atoms.
- Understanding the Lewis structure of SrBr2 is essential for predicting its molecular geometry and properties.
Step-by-Step Guide to Drawing the SrBr2 Lewis Structure
- Start by drawing the strontium atom as the central atom.
- Calculate the total number of valence electrons: 2 (Sr) + 2 x 7 (Br) = 16.
- Draw the electrons around the central atom: two valence electrons for strontium.
- Add the two bromine atoms, each with seven valence electrons.
- Form the ionic bonds between the strontium atom and the two bromine atoms.
Step 1: Draw the Strontium Atom
Start by drawing the strontium atom as the central atom.
Step 2: Calculate the Total Number of Valence Electrons
Calculate the total number of valence electrons: 2 (Sr) + 2 x 7 (Br) = 16.
Step 3: Draw the Electrons Around the Central Atom
Draw the electrons around the central atom: two valence electrons for strontium.
Step 4: Add the Bromine Atoms
Add the two bromine atoms, each with seven valence electrons.
Step 5: Form the Ionic Bonds
Form the ionic bonds between the strontium atom and the two bromine atoms.
Frequently Asked Questions
What is the molecular geometry of SrBr2?
+The molecular geometry of SrBr2 is linear, with the strontium atom in the center and the two bromine atoms on either side.
Why is SrBr2 an ionic compound?
+SrBr2 is an ionic compound because it is formed by the transfer of electrons between the strontium and bromine atoms, resulting in the formation of ions with opposite charges.
What is the electron configuration of strontium?
+The electron configuration of strontium is [Kr] 5s2.
By following these steps and understanding the Lewis structure of SrBr2, you’ll be able to better comprehend the properties and behavior of this ionic compound. Remember to practice drawing Lewis structures to become more proficient in chemistry.


