Structure Of Ph3
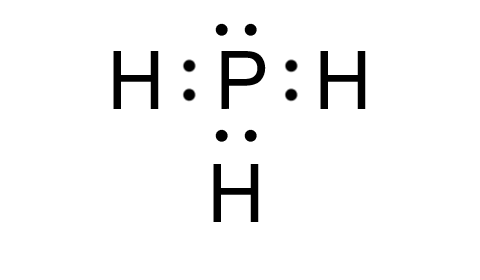
Phosphine, denoted by the chemical formula PH3, is a colorless, highly toxic, and flammable gas. It is one of the simplest phosphorus-containing compounds and has a significant presence in various chemical and industrial applications. Understanding the structure of PH3 is crucial for appreciating its properties and reactivity.
Molecular Geometry
The molecular geometry of PH3 is trigonal pyramidal. This geometry is a result of the molecule’s electronic structure, particularly the arrangement of electron pairs around the central phosphorus atom. In PH3, the phosphorus atom is bonded to three hydrogen atoms, and it also has a lone pair of electrons. The bonding pairs and the lone pair occupy the four sp3 hybrid orbitals of the phosphorus atom, leading to a tetrahedral arrangement of these electron pairs in space. However, because the lone pair is not visible and does not contribute to the molecular shape in the same way as bonding pairs, the actual shape of the molecule is trigonal pyramidal.
Bonding
The bonding in PH3 involves the overlap of atomic orbitals from phosphorus and hydrogen. Phosphorus, being in group 15 of the periodic table, has five valence electrons. It uses three of these electrons to form single bonds with three hydrogen atoms, which each contribute one electron to the bond. The remaining two electrons on phosphorus form a lone pair. The bonds between phosphorus and hydrogen are polar due to the difference in electronegativity between phosphorus and hydrogen. However, the polarity of the P-H bonds is not as pronounced as in other hydrides of more electronegative elements, such as oxygen (in water, H2O) or nitrogen (in ammonia, NH3), due to phosphorus’s lower electronegativity compared to these elements.
Comparison with Other Hydrides
PH3 is often compared with other simple hydrides, particularly NH3 (ammonia) and H2O (water), due to their similar molecular formulas and geometries. All three are pyramidal in shape due to the presence of a lone pair on the central atom (phosphorus, nitrogen, or oxygen). However, their physical and chemical properties vary significantly. For example, PH3 is much less polar than NH3 or H2O, which can be observed in its much lower boiling point and its lack of extensive hydrogen bonding in the liquid phase. This difference in polarity and resulting intermolecular forces is largely due to the lower electronegativity of phosphorus compared to nitrogen or oxygen, leading to less charge separation within the molecule.
Synthesis and Applications
Phosphine can be synthesized by the reaction of a metal phosphide (such as aluminium phosphide or zinc phosphide) with water or dilute acid. It has various applications, including its use as a fumigant for grains and soil and as a precursor for the synthesis of other phosphorus compounds. However, its high toxicity limits its handling and application.
Safety Considerations
Given its high toxicity, handling PH3 requires special precautions. Phosphine is highly toxic by inhalation, and its effects on the human body can be severe, including respiratory distress, neurological damage, and even death at high concentrations. It also poses significant environmental hazards, particularly to aquatic life.
Conclusion
In conclusion, the structure of PH3, with its trigonal pyramidal geometry, reflects the underlying electronic configuration of the phosphorus atom and influences its chemical and physical properties. Understanding this structure and its implications for reactivity and intermolecular interactions is crucial for safely handling and utilizing PH3 in various applications.
What is the molecular geometry of PH3?
+The molecular geometry of PH3 is trigonal pyramidal, resulting from the tetrahedral arrangement of electron pairs around the central phosphorus atom.
Why is PH3 less polar than NH3 or H2O?
+PH3 is less polar than NH3 or H2O due to the lower electronegativity of phosphorus compared to nitrogen or oxygen, leading to less charge separation within the PH3 molecule.
What are the primary hazards associated with PH3?
+The primary hazards associated with PH3 are its high toxicity by inhalation and its potential environmental impacts, particularly on aquatic life.

