Table Ir Spectroscopy
Infrared (IR) spectroscopy is a widely used analytical technique that provides valuable information about the molecular structure, functional groups, and chemical bonds present in a sample. IR spectroscopy is based on the principle that molecules absorb specific wavelengths of infrared radiation, resulting in a unique spectral fingerprint that can be used to identify and characterize the sample.
Introduction to IR Spectroscopy
IR spectroscopy involves measuring the absorption of infrared radiation by a sample as a function of wavelength or frequency. The infrared region of the electromagnetic spectrum spans from approximately 780 nanometers (nm) to 1 millimeter (mm), and IR spectroscopy typically focuses on the mid-infrared region (4000-400 cm-1). When a sample is exposed to infrared radiation, the molecules absorb specific wavelengths, causing the molecules to vibrate or rotate. These vibrations and rotations are characteristic of the molecular structure and functional groups present in the sample.
Instrumentation and Sampling Techniques
IR spectroscopy can be performed using various instrumentation and sampling techniques, including:
- Dispersive IR spectroscopy: This traditional technique uses a prism or grating to disperse the infrared radiation, and the sample is typically measured in transmission mode.
- Fourier transform IR (FTIR) spectroscopy: This modern technique uses an interferometer to measure the IR spectrum, providing higher sensitivity and faster analysis times.
- Attenuated total reflectance (ATR) spectroscopy: This technique uses a crystal to measure the IR spectrum, allowing for the analysis of solid and liquid samples with minimal sample preparation.
- Diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS): This technique measures the IR spectrum of a sample in reflectance mode, allowing for the analysis of powders and irregularly shaped samples.
Interpretation of IR Spectra
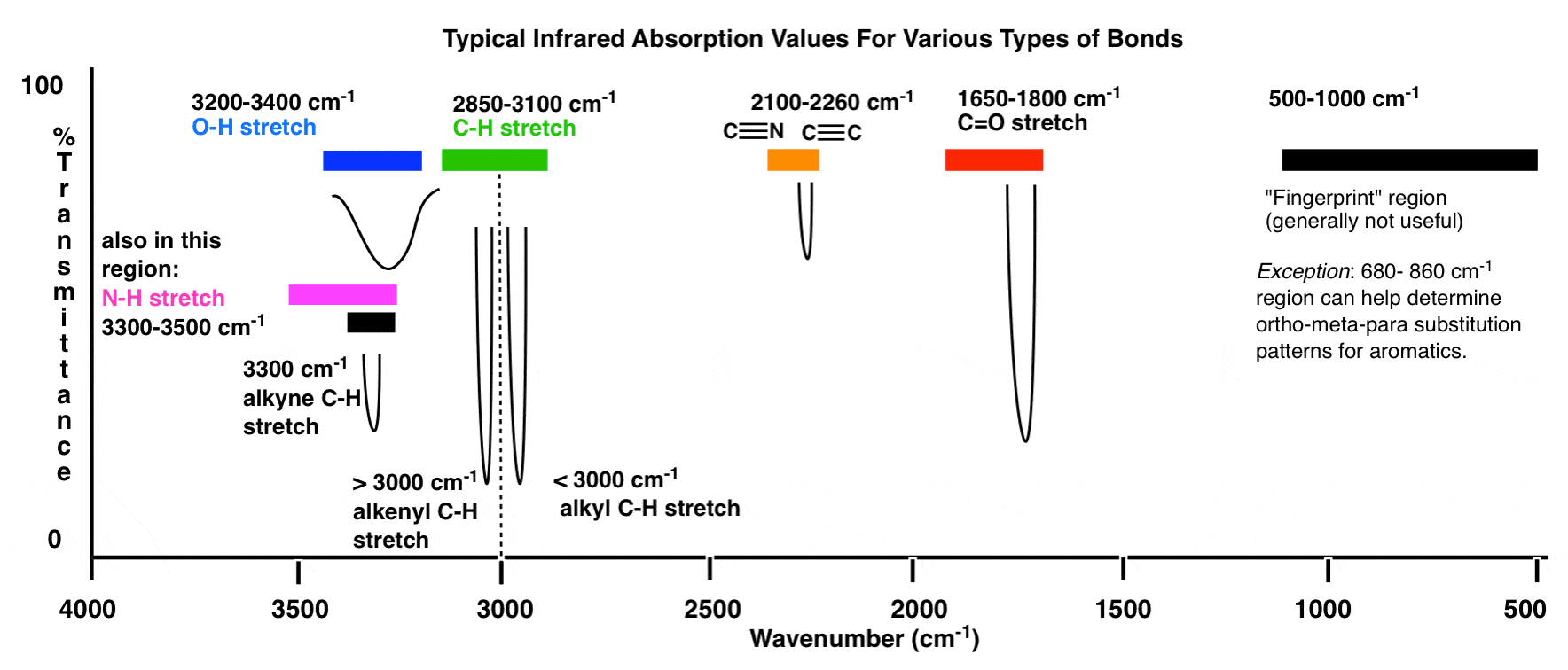
The interpretation of IR spectra involves assigning the observed absorption bands to specific molecular vibrations or functional groups. The most common functional groups and their corresponding IR absorption frequencies are:
- C-H stretching: 3000-2800 cm-1
- C=O stretching: 1800-1600 cm-1
- C-O stretching: 1300-1000 cm-1
- N-H stretching: 3500-3200 cm-1
- O-H stretching: 3600-3200 cm-1
Applications of IR Spectroscopy
IR spectroscopy has a wide range of applications in various fields, including:
- Chemical identification: IR spectroscopy is commonly used to identify unknown chemicals, verify the purity of substances, and detect contaminants.
- Pharmaceutical analysis: IR spectroscopy is used to analyze the molecular structure and purity of pharmaceutical compounds, as well as to detect counterfeit products.
- Food and beverage analysis: IR spectroscopy is used to analyze the chemical composition of food and beverages, including the detection of adulterants and contaminants.
- Environmental monitoring: IR spectroscopy is used to monitor environmental pollutants, such as volatile organic compounds (VOCs) and greenhouse gases.
IR spectroscopy is a powerful analytical technique that provides valuable information about the molecular structure and functional groups present in a sample. By understanding the principles and instrumentation of IR spectroscopy, researchers and scientists can unlock the full potential of this technique and apply it to a wide range of fields and applications.
Comparison of IR Spectroscopy with Other Analytical Techniques
IR spectroscopy is often compared with other analytical techniques, such as nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS). While each technique has its own unique advantages and limitations, IR spectroscopy is commonly preferred for its:
- Speed and simplicity: IR spectroscopy is typically faster and more straightforward to perform than NMR or MS.
- Cost-effectiveness: IR spectroscopy is often less expensive than NMR or MS, making it a more accessible option for many researchers and laboratories.
- Ease of interpretation: IR spectra are often easier to interpret than NMR or MS data, particularly for users without extensive training in spectroscopy.
| Technique | Advantages | Limitations |
|---|---|---|
| IR Spectroscopy | Fast, simple, cost-effective, easy to interpret | Limited to molecular structure and functional groups |
| NMR Spectroscopy | Provides detailed information on molecular structure and dynamics | Slow, complex, expensive, requires expertise |
| Mass Spectrometry | Provides detailed information on molecular weight and fragmentation patterns | Requires complex instrumentation and expertise, limited to certain types of samples |

Future Trends and Emerging Applications
IR spectroscopy is a continuously evolving field, with new instrumentation, techniques, and applications emerging regularly. Some of the future trends and emerging applications of IR spectroscopy include:
- Portable and handheld IR spectrometers: These instruments are designed for field-based analysis and provide rapid, on-site measurements.
- IR spectroscopy in biomedical research: IR spectroscopy is being increasingly used to study biomolecules, such as proteins and lipids, and to diagnose diseases.
- IR spectroscopy in art conservation: IR spectroscopy is used to analyze the chemical composition of artworks, such as paintings and sculptures, and to detect forgeries.
IR spectroscopy is a powerful and versatile analytical technique that provides valuable information about the molecular structure, functional groups, and chemical bonds present in a sample. By understanding the principles, instrumentation, and applications of IR spectroscopy, researchers and scientists can unlock its full potential and apply it to a wide range of fields and applications.
FAQs
What is IR spectroscopy, and how does it work?
+IR spectroscopy is an analytical technique that measures the absorption of infrared radiation by a sample, providing information about the molecular structure, functional groups, and chemical bonds present. The technique works by exposing the sample to infrared radiation and measuring the absorption of specific wavelengths.
What are the advantages and limitations of IR spectroscopy?
+The advantages of IR spectroscopy include its speed, simplicity, cost-effectiveness, and ease of interpretation. However, the technique is limited to providing information about the molecular structure and functional groups present in a sample, and it may not be suitable for all types of samples or applications.
What are the future trends and emerging applications of IR spectroscopy?
+The future trends and emerging applications of IR spectroscopy include the development of portable and handheld instruments, the use of IR spectroscopy in biomedical research, and the application of IR spectroscopy in art conservation. Additionally, IR spectroscopy is being increasingly used in various fields, such as pharmaceutical analysis, food and beverage analysis, and environmental monitoring.
