Valence Electrons In Cobalt
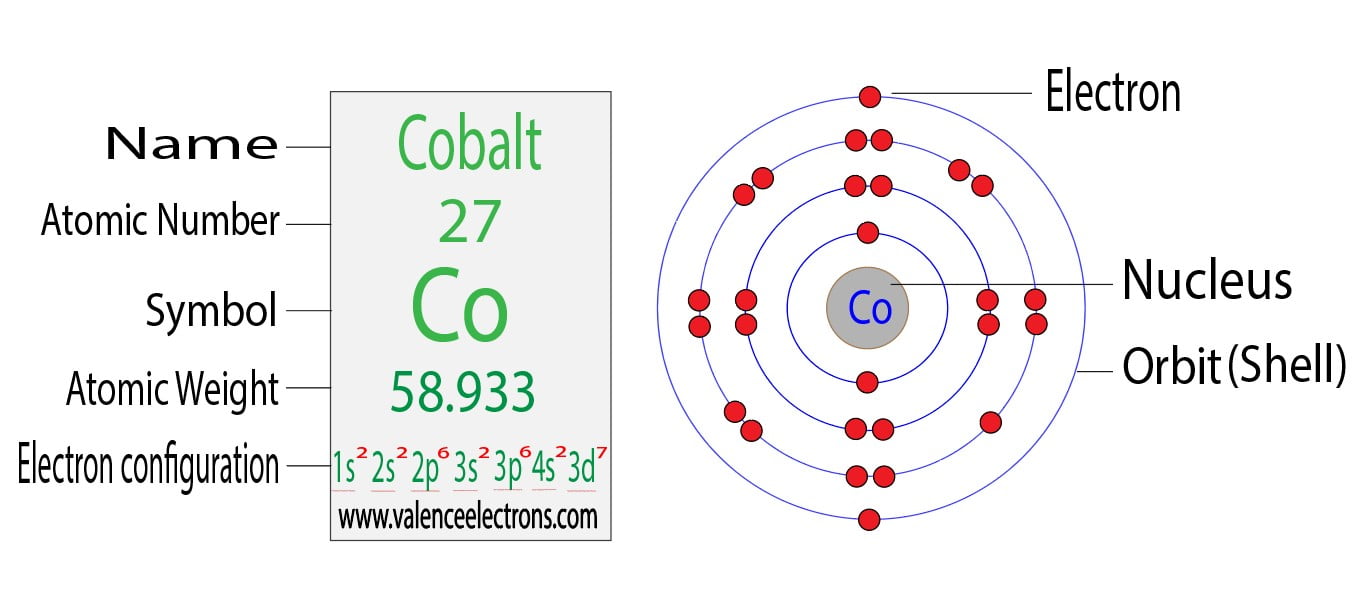
The atomic structure of cobalt, a transition metal with the atomic number 27, reveals a fascinating arrangement of electrons. To understand the valence electrons in cobalt, we must first delve into the basics of atomic structure and the electron configuration of this element.
Cobalt’s electron configuration is [Ar] 3d7 4s2, which tells us how its electrons are distributed across different orbitals. The valence electrons, responsible for the chemical properties of an element, are those found in the outermost shell of the atom. In the case of cobalt, the outermost shell is the fourth shell (n = 4), which contains the 4s orbital. However, because cobalt is a transition metal, its valence electrons are not limited to the outermost shell; they also include the electrons in the d orbitals of the third shell (n = 3), specifically the 3d orbitals.
The 3d orbitals in cobalt are filled with seven electrons, and the 4s orbital is filled with two electrons. These electrons - seven in the 3d orbitals and two in the 4s orbital - are considered the valence electrons of cobalt because they participate in chemical bonding. The ability of cobalt to form ions with different charges is directly related to the ease with which these valence electrons can be removed or shared.
One of the interesting aspects of transition metals like cobalt is their ability to exhibit variable valency, meaning they can form ions with different charges. Cobalt commonly forms Co2+ (cobalt(II)) and Co3+ (cobalt(III)) ions. The formation of these ions involves the removal of valence electrons. For Co2+, the ionization typically involves the removal of the two 4s electrons (leaving the 3d electrons intact), resulting in a d7 configuration. For Co3+, an additional electron is removed, usually one from the 3d orbitals, resulting in a d6 configuration.
The valence electrons of cobalt play a crucial role in its chemical reactivity and its ability to participate in various types of chemical bonds. In compounds, cobalt can exhibit a range of oxidation states, reflecting the flexibility of its valence electrons to adapt to different chemical environments. This adaptability is a hallmark of transition metals and underpins their widespread use in catalysis, materials science, and biological systems.
Understanding the valence electrons in cobalt is essential for appreciating its chemical behavior, including its reactions, the compounds it forms, and its physical properties. The unique electronic structure of cobalt, characterized by the presence of both 3d and 4s valence electrons, underlies its versatility in chemical and physical applications, from catalytic processes to magnetic materials.
Comparative Analysis of Valence Electrons in Nearby Transition Metals
To gain a deeper understanding of the valence electrons in cobalt, it’s useful to compare them with those of neighboring transition metals in the periodic table, such as iron (Fe) and nickel (Ni).
- Iron (Fe) has an electron configuration of [Ar] 3d6 4s2. Its valence electrons include the six electrons in the 3d orbitals and the two in the 4s orbital. Iron’s ability to form different ions, like Fe2+ and Fe3+, is akin to cobalt’s but reflects its specific electron configuration.
- Nickel (Ni), with an electron configuration of [Ar] 3d8 4s2, has eight electrons in its 3d orbitals and two in the 4s orbital as valence electrons. Nickel commonly forms a Ni2+ ion by losing its two 4s electrons.
This comparison highlights the gradual filling of the 3d orbitals across the first transition series and how each element’s unique electron configuration influences its chemical properties and the ions it can form.
Historical Evolution of Understanding Valence Electrons
The concept of valence electrons has evolved significantly over the history of chemistry. Early chemists recognized that certain elements could form compounds by either gaining, losing, or sharing electrons, but the underlying structure of the atom was not well understood. The discovery of the electron by J.J. Thomson in 1897 and subsequent models of the atom, including the Bohr model and quantum mechanical models, have provided a detailed understanding of electron configuration and valence electrons.
Future Trends in the Study of Valence Electrons
Advances in computational chemistry and materials science continue to rely heavily on the understanding of valence electrons in transition metals like cobalt. Research into new materials with novel properties, such as superconductors, nanomaterials, and magnetic materials, depends on precise control over the electronic structure of constituent atoms, including their valence electrons.
FAQ Section
What are valence electrons, and why are they important in chemistry?
+Valence electrons are the electrons in the outermost shell of an atom, which participate in chemical bonding. They are crucial for understanding the chemical properties and reactivity of an element.
How do the valence electrons of cobalt contribute to its ferromagnetic properties?
+The unpaired electrons in the 3d orbitals of cobalt, which are among its valence electrons, align their spins, resulting in a net magnetic moment that gives cobalt its ferromagnetic properties.
What is the difference between the valence electrons of cobalt and those of other transition metals like iron and nickel?
+The number of electrons in the 3d orbitals differs among these metals: cobalt has 7 electrons in its 3d orbitals, iron has 6, and nickel has 8. This difference affects their chemical properties and the ions they can form.
In conclusion, the valence electrons in cobalt play a pivotal role in its chemical and physical properties, including its ability to form different ions, its magnetic behavior, and its participation in various chemical compounds. Understanding these electrons is essential not only for appreciating the properties of cobalt but also for advancing research and applications in materials science, catalysis, and beyond.