What Guides Electron Configuration? Simplified Rules
Electron configuration is a crucial concept in chemistry that describes the distribution of electrons within an atom. It is guided by a set of principles and rules that help predict how electrons occupy the available orbitals. The main guides for electron configuration can be simplified into the following rules:
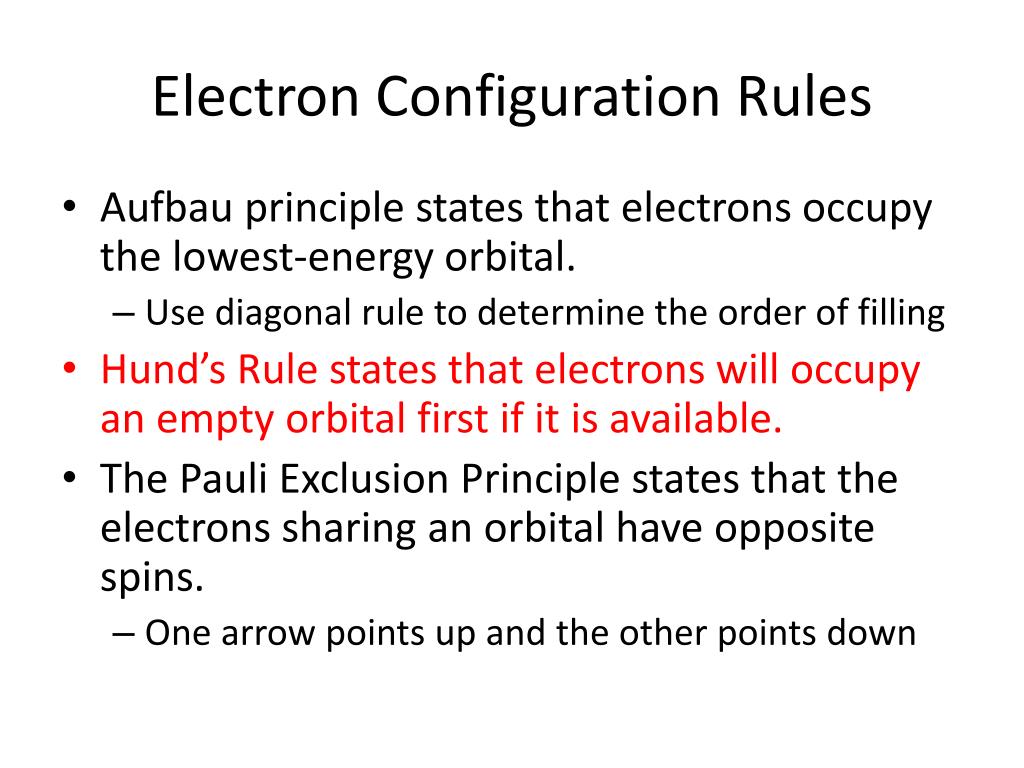
Aufbau Principle: This principle states that electrons occupy the lowest available energy levels. It implies that electrons fill the lowest-energy orbitals first before moving on to higher-energy ones. This principle helps in understanding how electrons are distributed among the various orbitals based on their energies.
Pauli Exclusion Principle: Proposed by Wolfgang Pauli, this principle asserts that no two electrons in an atom can have the same set of four quantum numbers. These quantum numbers are:
- n (principal quantum number): Describes the energy level or shell.
- l (azimuthal quantum number): Describes the orbital type (s, p, d, f).
- m_l (magnetic quantum number): Describes the orientation of the orbital in space.
- m_s (spin quantum number): Describes the spin of the electron, which can be either +1⁄2 or -1⁄2.
The Pauli Exclusion Principle ensures that each electron in an atom has a unique set of quantum numbers, which explains why an orbital can hold a maximum of two electrons, provided they have opposite spins.
Hund’s Rule of Maximum Multiplicity: This rule states that when filling degenerate orbitals (orbitals with the same energy), electrons occupy each orbital singly before pairing up. Furthermore, these singly occupied orbitals have parallel spins. This principle maximizes the spin multiplicity of the atom, which is a measure of the number of unpaired electrons. By doing so, electrons minimize their repulsion by staying apart as much as possible, while also following the Pauli Exclusion Principle.
The Madelung Rule (orAufbau + Pauli + Hund): Also known as the “n + l rule,” this combines the first three principles to provide a simplified method for determining the order in which orbitals are filled. According to this rule, electrons fill the orbitals in the order of increasing n + l value. When n + l values are the same for different orbitals, the orbital with the lower n value is filled first. This rule helps predict the electron configuration of atoms by guiding the sequence in which orbitals are occupied.
Understanding these rules is essential for predicting electron configurations, as they form the basis for understanding the structure of atoms and how they interact. Electron configuration is not only crucial for basic chemistry but also has implications in understanding the physical properties of materials, chemical reactivity, and the formation of chemical bonds.
To illustrate how these rules work in practice, consider the electron configuration of a simple atom like carbon ©. Carbon has six electrons. Following the Aufbau principle, these electrons occupy the lowest available energy levels. The first two electrons fill the 1s orbital, while the next two electrons occupy the 2s orbital. The remaining two electrons then fill the 2p orbitals, with each electron occupying a separate 2p orbital with parallel spins, as per Hund’s rule. This results in the electron configuration of 1s² 2s² 2p², which is a direct consequence of applying the simplified rules for electron configuration.
In practical terms, electron configuration has a wide range of applications, from materials science to pharmaceutical chemistry. For instance, understanding the electron configuration of atoms helps scientists predict the reactivity of elements and compounds, which is crucial in designing new materials and drugs.
By applying these simplified rules, chemists and physicists can better understand the atomic world and harness this knowledge to drive innovation and discovery.
The interplay between these principles forms the foundation of understanding electron configuration, allowing for a deeper insight into the atomic structure and its role in chemistry and beyond.
What is the primary principle guiding how electrons occupy energy levels in an atom?
+The Aufbau principle is the primary guide, stating that electrons fill the lowest available energy levels first.
Why can't two electrons in an atom have the same set of quantum numbers?
+This is due to the Pauli Exclusion Principle, which states that no two electrons can have the same set of four quantum numbers.
How do electrons fill degenerate orbitals according to Hund's Rule?
+Hund's Rule states that electrons occupy each degenerate orbital singly before any pairing occurs, with the spins of these singly occupied orbitals being parallel.
Understanding and applying these principles is crucial for a comprehensive grasp of electron configuration, facilitating a deeper appreciation of atomic and molecular behavior.


