What Is Carbon Dioxide: Ionic Or Covalent?
Carbon dioxide, denoted by the chemical formula CO2, is a compound that consists of one carbon atom covalently bonded to two oxygen atoms. The nature of the bond between carbon and oxygen in CO2 is primarily covalent. To understand why, let’s delve into the basics of chemical bonding and the specific characteristics of carbon dioxide.
Chemical Bonding: Ionic vs. Covalent
Chemical bonds are forces that hold atoms together in molecules. These bonds can be broadly categorized into two types: ionic and covalent.
Ionic Bonds: These occur when one or more electrons are transferred between atoms, leading to the formation of ions with opposite charges. The electrostatic attraction between these positively and negatively charged ions constitutes an ionic bond. Ionic bonds typically form between metals and nonmetals.
Covalent Bonds: These bonds involve the sharing of electron pairs between atoms. The sharing can be equal (as in nonpolar covalent bonds) or unequal (polar covalent bonds), depending on the electronegativities of the atoms involved. Covalent bonds are common among nonmetals.
Carbon Dioxide: A Covalent Compound
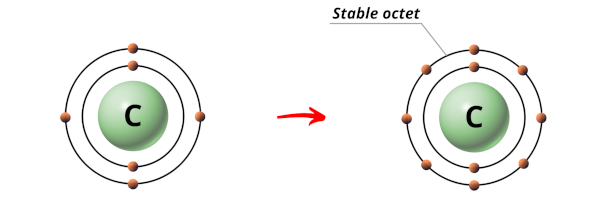
In the case of carbon dioxide (CO2), carbon © is a nonmetal, and oxygen (O) is also a nonmetal. When these elements combine to form CO2, they do so by sharing electrons. Specifically, the carbon atom shares its four valence electrons with two oxygen atoms in such a way that each oxygen atom shares two electrons with the carbon atom. This arrangement results in a stable molecule where the carbon atom achieves an octet configuration (having eight electrons in its outer shell), and each oxygen atom also achieves a stable configuration.
The bonds between carbon and oxygen in CO2 are polar covalent bonds due to the difference in electronegativity between carbon and oxygen. Oxygen is more electronegative than carbon, meaning it has a greater tendency to attract electrons. This results in a partial positive charge on the carbon atom and partial negative charges on the oxygen atoms. However, because the molecule is linear (with the carbon in the center and the oxygens on either side) and symmetrical, the dipole moments of the two C-O bonds cancel each other out, making CO2 a nonpolar molecule overall despite having polar covalent bonds.
Conclusion
In summary, carbon dioxide (CO2) is a covalent compound because it is formed through the sharing of electrons between carbon and oxygen atoms. The specific nature of these bonds is polar covalent due to the difference in electronegativity between carbon and oxygen. Understanding the covalent nature of CO2 is crucial for appreciating its chemical and physical properties, which play significant roles in various biological, environmental, and industrial processes.
Further Insights
For a deeper understanding of CO2 and its implications, let’s consider some additional aspects:
Greenhouse Gas: CO2 is a potent greenhouse gas. Its ability to absorb and emit infrared radiation makes it a significant contributor to the greenhouse effect, which is crucial for maintaining the Earth’s climate but also implicated in global warming when present in excess.
Biological Importance: Carbon dioxide is vital for plant photosynthesis, where it is converted into organic carbon compounds, releasing oxygen as a byproduct. This process is fundamental to life on Earth, supporting the food chain and influencing the carbon cycle.
Industrial Applications: CO2 has numerous industrial applications, including its use in carbonation of beverages, fire extinguishers, and as a supercritical fluid in extraction processes.
Practical Applications and Trends
The study of CO2’s properties and behaviors has led to various practical applications and ongoing research trends, including:
Carbon Capture and Utilization (CCU): Technologies aimed at capturing CO2 from power plants and industrial processes, followed by its conversion into valuable chemicals, fuels, or materials, offering a promising strategy for reducing emissions and creating new economic opportunities.
Climate Change Mitigation: Efforts to reduce CO2 emissions through the transition to renewable energy sources, enhancement of energy efficiency, and reforestation, among other strategies, are critical for mitigating climate change.
In conclusion, the covalent nature of carbon dioxide underpins its unique properties and its significant role in both natural and industrial contexts. Continual research and innovation are essential for addressing the challenges posed by CO2, from climate change to its potential as a resource in sustainable technologies.
Frequently Asked Questions
What type of bond does carbon dioxide exhibit?
+Carbon dioxide exhibits covalent bonds, specifically polar covalent bonds between the carbon and oxygen atoms due to the difference in their electronegativities.
Why is carbon dioxide important in biological processes?
+Carbon dioxide is crucial for plant photosynthesis, where it is used to produce glucose and oxygen. This process is foundational for life on Earth, supporting the food chain and influencing the carbon cycle.
What are some industrial applications of carbon dioxide?
+Carbon dioxide has various industrial applications, including the carbonation of beverages, use in fire extinguishers, and as a supercritical fluid in extraction processes. It is also being explored for its potential in carbon capture and utilization technologies.


