What Is Sodium Acetate Structure? A Clear Guide
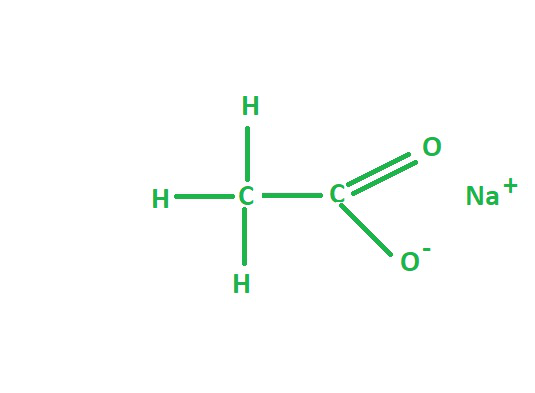
Sodium acetate, also known as sodium ethanoate, is a sodium salt of acetic acid and has the chemical formula CH3COONa. It is a white, crystalline powder that is highly soluble in water and has a wide range of applications in various industries, including food, pharmaceuticals, and textiles.
Chemical Structure
The chemical structure of sodium acetate consists of a sodium ion (Na+) and an acetate ion (CH3COO-). The acetate ion is derived from acetic acid (CH3COOH), where the hydrogen atom is replaced by a sodium ion. The resulting structure is a sodium cation and an acetate anion, which are held together by electrostatic forces.
The molecular structure of sodium acetate can be represented as:
CH3COONa
Where:
- CH3- is the methyl group
- COO- is the carboxylate group
- Na+ is the sodium ion
Physical Properties
Sodium acetate is a highly soluble compound in water, with a solubility of approximately 250 g/100 mL at 20°C. It is also soluble in ethanol and slightly soluble in ether. The compound has a melting point of approximately 324°C and a boiling point of 881°C.
Applications
Sodium acetate has a wide range of applications in various industries, including:
- Food industry: Sodium acetate is used as a food additive, where it serves as a preservative, flavor enhancer, and texture modifier.
- Pharmaceuticals: Sodium acetate is used as an excipient in the manufacture of pharmaceuticals, where it serves as a buffering agent and stabilizer.
- Textiles: Sodium acetate is used in the textile industry, where it serves as a dyeing agent and fabric finisher.
- Laboratory applications: Sodium acetate is used in laboratory settings as a buffer solution, where it helps to maintain a stable pH.
Preparation
Sodium acetate can be prepared by the reaction of acetic acid with sodium hydroxide:
CH3COOH + NaOH → CH3COONa + H2O
This reaction is a neutralization reaction, where the acid (acetic acid) reacts with the base (sodium hydroxide) to form the salt (sodium acetate) and water.
In conclusion, sodium acetate is a highly versatile compound with a wide range of applications in various industries. Its chemical structure consists of a sodium ion and an acetate ion, which are held together by electrostatic forces. The compound has a number of physical properties that make it highly soluble in water and other solvents, and it is prepared by the reaction of acetic acid with sodium hydroxide.
Sodium Acetate vs. Other Salts
Sodium acetate is just one of many salts that can be derived from acetic acid. Other salts include potassium acetate, calcium acetate, and magnesium acetate, among others. Each of these salts has its own unique properties and applications, and they are used in a wide range of industries, from food and pharmaceuticals to textiles and laboratory settings.
| Salt | Chemical Formula | Applications |
|---|---|---|
| Sodium Acetate | CH3COONa | Food, pharmaceuticals, textiles, laboratory applications |
| Potassium Acetate | CH3COOK | Food, pharmaceuticals, laboratory applications |
| Calcium Acetate | (CH3COO)2Ca | Food, pharmaceuticals, textiles, construction |

Conclusion
In conclusion, sodium acetate is a highly versatile compound with a wide range of applications in various industries. Its chemical structure consists of a sodium ion and an acetate ion, which are held together by electrostatic forces. The compound has a number of physical properties that make it highly soluble in water and other solvents, and it is prepared by the reaction of acetic acid with sodium hydroxide. Whether you are working in the food industry, pharmaceuticals, textiles, or laboratory settings, sodium acetate is a highly useful compound that can help you achieve your goals.
What is the chemical formula of sodium acetate?
+The chemical formula of sodium acetate is CH3COONa.
What are the physical properties of sodium acetate?
+Sodium acetate is a white, crystalline powder that is highly soluble in water. It has a melting point of approximately 324°C and a boiling point of 881°C.
What are the applications of sodium acetate?
+Sodium acetate has a wide range of applications in various industries, including food, pharmaceuticals, textiles, and laboratory settings.


