What Is The Difference Between Fractional Distillation And Simple Distillation

Introduction
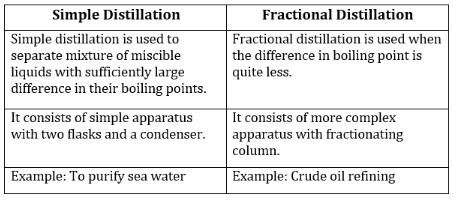
Distillation is a fundamental technique in chemistry used to separate mixtures of liquids based on differences in their boiling points. While both fractional distillation and simple distillation achieve this goal, they differ significantly in their methods, efficiency, and applications. Understanding these differences is crucial for selecting the appropriate technique for specific separation tasks.
Simple Distillation: A Basic Overview
Simple distillation is the most straightforward form of distillation. It involves heating a liquid mixture to its boiling point, vaporizing the more volatile component, and then condensing the vapor back into a liquid. This process is ideal for separating mixtures where one component has a significantly lower boiling point than the other.
Simple distillation is effective for separating mixtures like water and ethanol, where the boiling points differ by at least 25°C. However, it is inefficient for separating components with close boiling points.
Fractional Distillation: A More Sophisticated Approach
Fractional distillation is an advanced technique designed to separate mixtures of liquids with boiling points that are relatively close. It achieves higher purity by repeatedly vaporizing and condensing the components within a fractionating column.
Fractional distillation is widely used in industries such as petroleum refining, where crude oil is separated into various components like gasoline, diesel, and kerosene.
Comparative Analysis
To better understand the differences, let’s compare the two techniques in a structured manner:
| Aspect | Simple Distillation | Fractional Distillation |
|---|---|---|
| Efficiency | Low for mixtures with close boiling points | High, even for mixtures with close boiling points |
| Equipment | Basic setup with no fractionating column | Requires a fractionating column |
| Applications | Separating mixtures with large boiling point differences (e.g., water and ethanol) | Separating complex mixtures with close boiling points (e.g., crude oil) |
| Purity of Product | Moderate purity | High purity |
Historical Evolution
Distillation techniques have evolved over centuries. Simple distillation dates back to ancient civilizations, where it was used for purifying water and producing alcoholic beverages. Fractional distillation emerged later, with significant advancements during the Industrial Revolution, particularly in the petroleum industry.
Practical Applications
- Simple Distillation: Used in laboratories for basic separations and in small-scale production of distilled water or ethanol.
- Fractional Distillation: Essential in large-scale industries such as petrochemicals, pharmaceuticals, and beverage production.
Myth vs. Reality
Myth: Fractional distillation is always better than simple distillation.
Reality: The choice depends on the mixture’s properties and the desired purity. Simple distillation is sufficient for mixtures with large boiling point differences, while fractional distillation is necessary for complex mixtures.
Future Trends
Advancements in distillation technology, such as the use of membrane distillation and automated systems, are enhancing efficiency and reducing energy consumption. These innovations are likely to further differentiate the applications of simple and fractional distillation.
FAQ Section
Can simple distillation separate components with close boiling points?
+
No, simple distillation is ineffective for separating components with close boiling points. Fractional distillation is required for such mixtures.
Why is a fractionating column necessary in fractional distillation?
+
The fractionating column provides multiple stages of vaporization and condensation, allowing for precise separation of components with similar boiling points.
What industries commonly use fractional distillation?
+
Fractional distillation is widely used in the petroleum, pharmaceutical, and chemical industries for separating complex mixtures.
Is fractional distillation more energy-intensive than simple distillation?
+
Yes, fractional distillation typically requires more energy due to the repeated vaporization and condensation processes in the fractionating column.
Conclusion
While both fractional and simple distillation are essential techniques for separating liquid mixtures, they serve different purposes. Simple distillation is suitable for basic separations with large boiling point differences, whereas fractional distillation excels in handling complex mixtures with close boiling points. Understanding these differences ensures the selection of the most appropriate method for specific applications, whether in a laboratory or industrial setting.
Key Takeaway: The choice between simple and fractional distillation hinges on the boiling point differences of the mixture components and the desired purity of the final product.