Nickel Valence Electrons
Nickel, a lustrous, silvery-white metal with the atomic number 28, plays a crucial role in various industrial and technological applications. Its unique chemical properties, particularly its valence electrons, make it an essential element in alloys, batteries, and catalysis. Understanding nickel’s valence electrons is fundamental to grasping its reactivity, bonding behavior, and applications in different fields.
Electronic Configuration and Valence Electrons
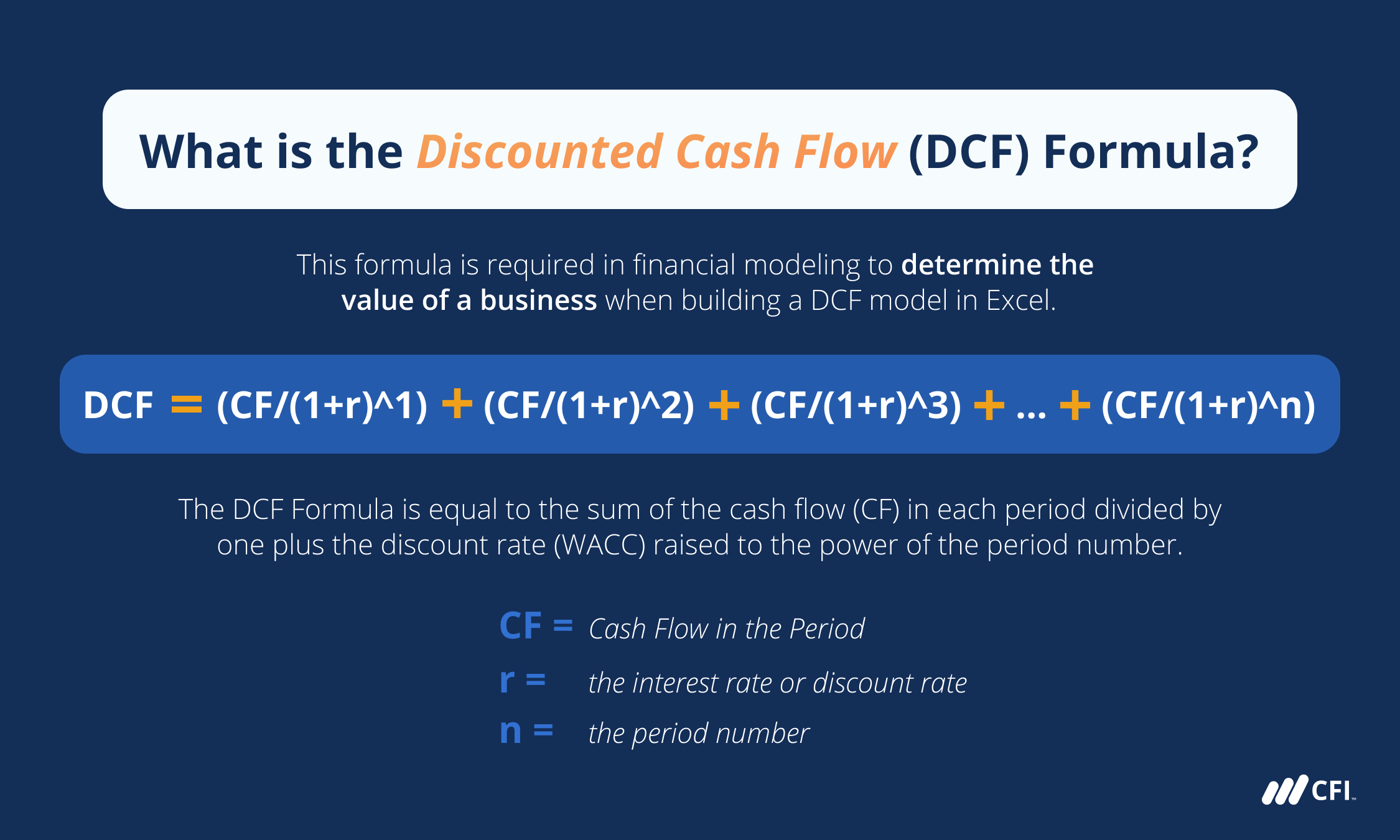
To determine nickel’s valence electrons, we must first examine its electron configuration. Nickel’s atomic number is 28, meaning it has 28 electrons. The electron configuration of nickel is:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸
In this configuration:
- The 1s, 2s, and 2p orbitals are filled, accounting for 10 electrons.
- The 3s and 3p orbitals are also filled, adding another 8 electrons.
- The remaining 10 electrons occupy the 4s and 3d orbitals.
The valence electrons are typically found in the outermost shell, which in nickel’s case is the 4s and 3d orbitals. However, the concept of valence electrons can be more complex for transition metals like nickel due to the involvement of d-orbitals.
Oxidation States and Chemical Behavior
Nickel’s valence electrons enable it to form various compounds and participate in chemical reactions. The most common oxidation states of nickel are:
- +2 (Ni²⁺): In this state, nickel loses two electrons from its 4s orbital, resulting in a stable electron configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 3d⁸. Examples of compounds include nickel(II) chloride (NiCl₂) and nickel(II) sulfate (NiSO₄).
- +3 (Ni³⁺): Less common, this state involves the loss of three electrons, two from the 4s orbital and one from the 3d orbital. An example is nickel(III) oxide (Ni₂O₃).
Applications of Nickel’s Valence Electrons
Nickel’s unique valence electron configuration and resulting chemical properties make it valuable in numerous applications:
- Alloys: Nickel is a key component in stainless steel, providing corrosion resistance and strength. It is also used in superalloys for high-temperature applications, such as jet engines and gas turbines.
- Batteries: Nickel-based batteries, like nickel-cadmium (NiCd) and nickel-metal hydride (NiMH), rely on nickel’s ability to undergo reversible redox reactions.
- Catalysis: Nickel catalysts are used in various industrial processes, including hydrogenation reactions and petrochemical refining.
Historical Context and Discovery
Nickel was discovered in 1751 by Swedish chemist Axel Fredrik Cronstedt, who initially mistook it for copper ore. The name “nickel” comes from the German word “kupfernickel,” meaning “false copper.” Historically, nickel has been used in coins, armor, and as a component in various alloys.
Future Implications and Research
Ongoing research focuses on optimizing nickel’s properties for advanced applications, such as:
- Energy storage: Improving nickel-based battery technologies for electric vehicles and renewable energy systems.
- Catalysis: Developing more efficient nickel catalysts for sustainable chemical processes.
- Materials science: Exploring nickel-based materials for high-temperature and corrosion-resistant applications.
FAQ Section
How many valence electrons does nickel have?
+Nickel has 2 valence electrons in its 4s orbital, but due to the involvement of 3d orbitals, it can exhibit variable valence states, commonly +2 and +3.
Why does nickel exhibit multiple oxidation states?
+Nickel's 4s and 3d orbitals are close in energy, allowing electrons to be shared between them. This results in variable oxidation states, such as +2 and +3.
div>What are the primary applications of nickel?
+Nickel is widely used in alloys (stainless steel, superalloys), batteries (NiCd, NiMH), and as a catalyst in various industrial processes.
How is nickel extracted and processed?
+Nickel is primarily extracted from nickel sulfide ores through roasting, smelting, and refining processes. Laterite ores are processed using hydrometallurgical methods.
What are the environmental impacts of nickel mining?
+Nickel mining can lead to soil erosion, water pollution, and habitat destruction. However, sustainable practices and recycling efforts are mitigating these impacts.
Conclusion
Nickel’s valence electrons are fundamental to its chemical behavior and diverse applications. From its role in alloys and batteries to its use in catalysis, nickel’s unique properties continue to drive innovation across industries. As research progresses, nickel is poised to play an even more significant role in addressing global challenges, such as energy storage and sustainable materials development. By understanding nickel’s valence electrons, we gain valuable insights into its reactivity, bonding, and potential for future advancements.